Browse through our Medical Journals...
 Focus
on
Risperidone
Focus
on
Risperidone
Dr Ben Green
MRCPsych,
University of Liverpool
| Introduction | Dosage Regime | Structure | Pharmacokinetics |
| Indications | Clinical Efficacy | Health Economics | Adverse effects |
| Comparisons | Binding profile | Conclusion |
Keywords: Schizophrenia - risperidone - antipsychotic - neuroleptic - atypical - pharmacology
Summary
Risperidone is a relatively new antipsychotic available world-wide since the early 1990s. It has been characterised as atypical, but shares some of the extrapyramidal side effect profile of the earlier antipsychotics, particularly at higher doses.
Introduction
Risperidone has been developed by Janssen-Cilag. It is a novel antipsychotic with dopaminergic and serotonergic effects. Risperidone is available in tablet and liquid form. A depot formulation is available (Risperdal Consta®).
The main pharmacological activities of risperidone include serotonin 5-HT2 receptor blockade and dopamine D2 antagonism (Megens et al, 1994). After oral administration of 1 mg of risperidone 5-HT2 receptor occupancy is about 60% and D2 dopamine receptor occupancy in the striatum is about 50% (Nyberg et al, 1993). In common with other antipsychotics, risperidone enhances prolactin release, but some central effects such as catalepsy and blockade of motor activity occur at high doses only. Risperidone is 4-10 times less potent than the conventional antipsychotic haloperidol as a central D2 antagonist in rats. Interaction with dopamine D1 receptors occurs only at very high concentrations. The pharmacological profile of risperidone includes interaction with histamine H1 and alpha-adrenergic receptors but the compound does not interact significantly with cholinergic receptors.The drug has good activity against various symptoms and signs associated with schizophrenia (Marder, Davis & Chouinard, 1997). Compared to conventional antipsychotics such as haloperidol risperidone produces some significantly better results according to Positive and Negative Syndrome Scale (PANSS) scores. Marder et al's study (1997) factor analysed the PANSS scores and produced five dimensions; negative symptoms, positive symptoms, disorganized thought, uncontrolled hostility/excitement, and anxiety/depression. The study looked at 513 patients intwo double-blind trials. Symptom reductions in PANSS factor scores from baseline to treatment at weeks 6 and 8 were significantly greater in patients receiving 6-16 mg/day of risperidone than in patients receiving placebo or haloperidol.The advantages of risperidone were greatest for negative symptoms, uncontrolled hostility/excitement, and anxiety/depression.
Meta-analysis from available randomised, double-masked, comparative trials of risperidone and haloperidol in patients with schizophrenia treated for at least 4 weeks at recommended doses (Davies et al, 1998). Six out of nine trials met all criteria for inclusion in the meta-analysis which showed that in patients with chronic schizophrenia, risperidone therapy is associated with significantly higher response rates, significantly less prescribing of anticholinergic medication, and significantly lower treatment dropout rates than haloperidol.
Dosage Regime
The drug is available in 0.25, 0.5, 1, 2, 3 and 4 mg tablets.
In adults the suggested initial dose schedule for risperidone is to titrate doses upward from 1 mg twice daily, to 2mg b.d. the next day and 3 mg b.d. the day after to achieve a dose of 4-6mg daily. However, there has been recent work recommending a less rapid titration (over 6 days to a week) and that the dose increments consist of 0.5-2 mg/day (Luchins et al, 1998). The starting dose in the eldelry is 0.5 mg b.d. with 0.5 mg increements to 2 mg b.d.
The conventional dosing regime is twice daily, although there has been recent interest in a once-daily regime. A recent double-blind 6-week study of 211 patients with acute exacerbation were randomly assigned to receive risperidone at 8 mg once daily or 4 mg twice daily. The study demonstrated little clinical difference between 8mg given once daily and 4mg given twice daily (Nair, 1998).In terms of switching patients over from conventional antipsychotics to risperidone about sixty per cent of patients can have their current neuroleptics stopped and risperidone started immediately together with a gradual withdrawal of anticholinergic treatments (Kirov et al, 1997). ). This strategy is understandably more successful for who previously received conventional antipsychotics as depot medication.
Nyberg et al (1999) have suggested 4mg/day as the best minimal dosage regime based on PET receptor occupancy studies.They comment that treatment with risperidone, 6 mg/day, is likely to induce unnecessarily high D2 receptor occupancy, with a consequent extrapyramidal side effects. They found that high 5-HT2A receptor occupancy did not prevent extrapyramidal side effects completely. The authors previously suggested an optimal interval for D2 receptor occupancy of 70%-80%. To achieve this, they suggested risperidone, 4 mg/day, as a suitable initial dose for antipsychotic effect with a minimal risk of extrapyramidal side effects in most patients.
It is the Serotonin 5-HT 2A and Dopamine D2 receptor occupancy that seem to provide the therapeutic effects of risperidone. In vivo PET studies have found that the Serotonin 5-HT 2A and Dopamine D2 receptor occupancy rates are 60% in the neocortex and 50% in the striatum for the respective receptor types (Nyberg et al, 1993). The Serotonin 5-HT 2A binding in the cortex disinhibits the mesocortical dopamine system , resulting in an increase in dopamine transmission in this pathway and is thought to account for the clinical efficacy against negative and affective symptoms.
Structure
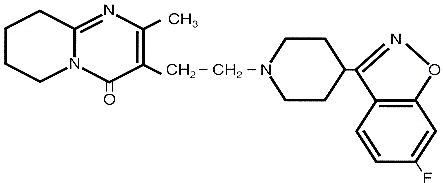
Risperidone (R64 766) is a benzisoxazole derivative whose molecular formula is C23 H27FN4O2. Figure One is a diagram of the risperidone molecule.
Clinical Indications
As far as the manufacturers are concerned risperidone is indicated for acute and chronic schizophrenic psychoses and other psychotic conditions with positive and negative symptoms. It is also indicated for affective symptoms associated with schizophrenia (ABPI, 1998). There is ample evidence of its efficacy, (Song, 1997).
There is some evidence that risperidone is useful to some extent in reducing aggression in schizophrenia, (Buckley et al, 1997) although possibly not more effectively than typical antipsychotics (Beck et al, 1997).Delusions of infestation appear responsive in small series studies, (De Leon et al, 1997).There is some evidence for its usefulness as an adjunctive therapy in acute bipolar affective disorder in outpatients (Ghaemi et al, 1997) and in bipolar disorder when followed up over a six month period (Ghaemi & Sachs, 1997). It has been postulated that there is dopaminergic mediation as well as a serotonergic mediation for some obsessive and related disorders e.g. Tourette's syndrome. Risperidone has therefore also been used to augment SSRIs in obsessive compulsive disorder (Saxena et al, 1996, Stein et al, 1997). In Saxena et al's study about eighty per cent of patients improved within three weeks of the addition of risperidone. In a series of 22 patients risperidone was found to be effective in 58 %, (Bruun & Budman, 1996). In affective psychoses, risperidone is probably not useful as a single therapeutic agent, that is to say it does not seem to replace an antipsychotic/antidepressant combination (Muller et al, 1998).Risperidone has been found useful in adolescent schizophrenia (Armenteros et al, 1997) and in children with autistic/pervasive developmental disorders (McDougle et al, 1997, Findling et al, 1997).There has been considerable interest and study of its use in the elderly. There has been documented beneficial use in dementia with persistent voacalisations (Kopala & Honer, 1997), and in demented people with Parkinson's (Workman et al, 1997). Katz et al (1999) found that 1 mg/day was useful in controlling aggression in severe dementia.There is ample study and anecdotal evidence for the use of risperidone in liaison psychiatry, uses include delirium (Siphimalani & Masand, 1997, Furmaga et al, 1997), HIV related psychotic disorders (Singh et al, 1997)
Pharmacokinetics
Risperidone is rapidly and very well absorbed after administration orally; less than 1% is excreted unchanged in the faeces (Heykants et al, 1994). Risperidone is 90% plasma protein bound (Borison et al, 1994). The principal metabolite is 9-hydroxyrisperidone. Hydroxylation of risperidone is subject to the same genetic CYP2D6-related polymorphism as for debrisoquine and dextromethorphan. In poor metabolizers the half-life of risperidone was about 20 hours compared with about 3 hours in extensive metabolizers (Huang et al, 1993). However, because the pharmacology of 9-hydroxyrisperidone is very similar to that of risperidone, the half-life for the "active moiety" (risperidone +9-hydroxyrisperidone) was found to be about 20 hours in poor metabolizers. Risperidone exhibits linear elimination kinetics. Steady state is reached within 1 day for risperidone and within 5 days for the active fraction.
Health Economics
There has been considerable debate about the direct medication costs and the cost efficacy of novel antipsychotics such as risperidone. There are health economic studies that indicate reduced indirect costs associated with poor compliance, such as repeated acute ward admissions, but also perhaps a shift in resources towards community care (day hospital places and the like). Viale et al (1997) investigated costs associated with risperidone and found days in acute care inpatient facilities were reduced by 26 percent, and days in residential treatment were reduced by 57 percent. These reductions were accompanied by an increase in the use of lower-cost services, such as community and day hospital treatment.
Risperidone and other atypical antipsychotics appear to promote a higher quality of life compared to conventional antipsychotics. Physical well being, social life and everyday life have also been rated higher in a comparative study using risperidone (Franz et al, 1997). In one evaluation, compared to haloperidol, a conventional antipsychotic, risperidone-treated patients obtained more than twice as many quality-adjusted years as haloperidol patients and in addition risperidone was found to be cost-effective, (Chouinard & Albright, 1997). A U.S. evaluation of costs, including indirect ones came up with figures for treatment with haloperidol and risperidone (Keks, 1997). A robust decision-analytic model of schizophrenia suggested that the overall 1997 cost of treating a patient with risperidone would be $11,772.00 per year compared with $13,622.00 per year for haloperidol and that the cost per response is even more favourable for risperidone; $14,599.00 versus $23,040.00.Guidelines issued by the Australian Pharmaceutical Benefits Advisory Committee have been used to construct a model for comparing the cost-effectiveness of risperidone and haloperidol over a 2-year period in patients with chronic schizophrenia (Davies et al, 1998). Cost-effectiveness was determined by using decision-analytic modelling. The analyses included all significant direct costs (i.e., hospital costs; outpatient costs; and the cost of drugs, the services of health care professionals, and government-subsidised hostel accommodation). The cost for a given outcome was the sum of costs for all scenarios leading to that outcome. Cost-effectiveness was expressed as the total cost per favourable outcome, i.e. where the patient was responding to treatment at the end of the 2-year period. The probability of a patient experiencing a favourable outcome at the end of 2 years was 78.9% for risperidone versus 58.9% for haloperidol. The total cost of treatment for 2 years was $15,549.00 for risperidone versus $18,332.00 for haloperidol. The expected cost per favourable outcome was $19,709.00 for risperidone and $31,104.00 for haloperidol. Risperidone was more cost-effective than haloperidol and therefore was "dominant" in pharmacoeconomic terms because it produced a higher proportion of favourable outcomes at lower cost.
Adverse effects
Common side effects include insomnia (about 8% of patients), weight gain, agitation, anxiety and headache. Less frequent side effects include somnolence, tiredness, dizziness, poor concentration, nausea, and dysfunctions of erection, ejaculation and orgasm.
Orthostatic hypotension can occur particularly initially. Prolactin rises can induce galactorrhoea and gynaecomastia along with disturbances of the menstrual cycle and amenorrhoea, (Kim, Kim & lee, 1999). Prolactin rises are also documented in males (Markianos Hatzimanolis & Lykouras, 1999)Risperidone appears to have less potential for causing EPS than conventional antipsychotics and as such may be more suitable as a maintenance antipsychotic than conventional dopamine-blockers (Umbricht & Kane, 1996, Kopala et al, 1997). In a study completed by over two hundred chronic schizophrenic patients by Simpson & Lindenmayer (1997) the severity of EPS in a risperidone treated group as measured by the Extrapyramidal Symptom Rating Scale (ESRS) score did not differ significantly differ from placebo group. There was a linear relationship between mean change scores and increasing risperidone dose on 4 of the 12 ESRS subscales. However, even at 16 mg/day of risperidone, mean change scores were lower than in a group treated with haloperidol group. A linear relationship between increasing risperidone dose and use of antiparkinsonian medications was also apparent.In a study of more than a hundred elderly patients, often with co-morbid medical conditions risperidone was found to be a safe and effective antipsychotic (Zarate et al, 1997). In this study there were adverse events in 32% of the patients. These adverse events included hypotension (29%), extrapyramidal effects (11%), symptomatic orthostasis (10%), cardiac arrest (1.6%) with fatality (0.8%), and delirium (1.6%).There are several reports of neuroleptic malignant syndrome with risperidone, (Singer et al, 1995, Bonwick et al, 1996, Gleason, & Conigliaro, 1997, Bajjoka et al, 1997). There is at least one recent report associating a year's exposure to risperidone with tardive dyskinesia, although this was in a patient who had previously been exposed to classical antipsychotics (Silberbauer, 1998). There have been reports of priapism associated with risperidone (Tekell, Smith & Silva, 1995). There is at least one case report of risperidone causing sudden cardiac death (Ravin & Levinson, 1997). Annual adverse event monitoring in the UK has shown that the relative risk with risperidone compared to other antipsychotics is decreased (Tooley & Zuiderwijk, 1997). Annual reporting rates for tardive dyskinesia were 0.0006% of patients and neuroleptic malignant syndrome 0.017%. EPS reports were made for 0.2% of patients. It is unlikely though that all adverse events are reported, and these probably represent an underestimate of the true incidence.A variant tardive abnormal movement, rabbit syndrome, has been described with risperidone, (Levin & Heresco Levy, 1999). Rabbit syndrome is a rare side effect of chronic neuroleptic administration characterized by rapid, fine, rhythmic movements of the mouth along a vertical axis.Overdoses of up to 360 mg have been reported. Problems include sedation, tachycardia, hypotension and EPS. QT prolongation may occur on ECG. One of the first overdoses with risperidone was noted in 1993. ECG abnormalities were recorded, but there was no fatality, (Brown et al, 1993). Nevertheless fatalities have been reported, (Springfield & Bodiford, 1996).There may be interactions with carbamazepine, which decreases the plasma levels of the antipsychotic fraction of risperidone. Similar drugs that induce hepatic enzymes may have the same effect. Phenothiazines, tricyclic antidepressants, fluoxetine, haloperidol and some beta blockers can increase plasma concentrations of risperidone.No teratogenic effect has been yet noted, but caution should be exercised before prescribing in pregnancy. Risperidone is excreted in milk in animal studies. Women receiving risperidone should not therefore breast feed.
Comparison with other antipsychotics
Extrapyramidal side effects, hyperprolactinemia, and sexual dysfunction are significantly more frequent with risperidone than olanzapine (Tran et al, 1997).
Patients who are treatment resistant as conventionally defined may probably be better treated with clozapine. However there is at least one recent study that suggests that a trial of risperidone may be worthwhile in conventional antipsychotic treatment resistance (Bondolfi et al, 1998). There appears to be little benefit to switching patients non-responsive to clozapine to risperidone, (Still et al, 1996). Clozapine is more effective than risperidone at reducing positive symptoms in resistant patients and is less likely to disturb the prolactin system (Breier et al, 1999).Compared to other antipsychotics it has moderate tendencies to cause weight gain. It is less likely to cause weight gain than olanzapine or clozapine (Wirshing et al, 1999)
Conclusion
Risperidone is one of a new generation of antipsychotic drugs with relatively fewer side effects and equal efficacy for florid 'positive' symptoms. The additional serotonergic actions of these new antipsychotics deliver further efficacy against 'negative' and affective symptoms of schizophrenia. There are however differences in the side effect profiles of the new antipsychotics and in some instances risperidone performs less well than others especially where higher doses are involved, inducing EPS. Despite their higher direct costs such antipsychotics, including particularly risperidone, have interesting economic arguments in their favour compared to earlier antipsychotics.
Correspondence:
Dr Ben Green, MRCPsych, Hon. Senior Lecturer in Psychiatry,
Division of Psychiatry
Royal Liverpool University Hospital,
The University of Liverpool, UK.
Email: bengreen@liverpool.ac.uk
References
Association of British Pharmaceutical Industries (1998) ABPI Compendium of Data Sheets. Summaries of Product Characteristics 1998-99.. London, ABPI.
Armenteros, JL, Whitaker, A H, Welikson, M, Stedge, J, Gorman, J. (1997) Risperidone in adolescents with schizophrenia: an open pilot study. J.Am. Acad. Child Adolesc. Psychiatry. 36(5): 694-700.Bajjoka, I, Patel, T, O'Sullivan, T. (1997) Risperidone-induced neuroleptic malignant syndrome. Ann.Emerg.Med. 30(5): 698-700Beck, N C et al. (1997) Risperidone in the management of violent, treatment-resistant schizophrenics hospitalized in a maximum security forensic facility. J.Am.Acad.Psychiatry Law. 25(4): 461-8.Bondolfi et al, (1998) Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomized double-blind study. The Risperidone Study Group. Am.J.Psychiatry. 155(4): 499-504.Bonwick, R J, Hopwood, M J, Morris, PL. (1996) Neuroleptic malignant syndrome and risperidone: a case report.. Aust. N.Z.J. Psychiatry 30(3): 419-21.
Borison, R. (1994) Risperidone: pharmacokinetics. J.Clin.Psychiatry Monograph 12 (2) 46-48.
Breier AF, Malhotra AK, Su TP. et al. (1999) Clozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine response. Am J Psychiatry 156:2, 294-8Brown, K. (1993) Overdose of risperidone. Ann. Emerg. Med. 22(12): 1908-10.
Buckley, F, et al (1997) Aggression and schizophrenia: efficacy of risperidone. J. Am. Acad. Psychiatry Law. 25(2): 173-81.Bruun, R D, Budman, C L (1996) Risperidone as a treatment for Tourette's syndrome. J.Clin.Psychiatry. 57(1): 29-31.Chouinard, G, Albright, P S. (1997) Economic and health state utility determinations for schizophrenic patients treated with risperidone or haloperidol. J. Clin. Psychopharmacol. 17(4): 298-307.Davies, A et al. (1998) Risperidone versus haloperidol: I. Meta-analysis of efficacy and safety. Clin. Ther. 20(1): 58-71Davies, A et al. (1998) Risperidone versus haloperidol: II. Cost-effectiveness. Clin.Ther. 20(1): 196-213.De-Leon, O A, Furmaga, K M, Canterbury, A L, Bailey, L G. (1997) Risperidone in the treatment of delusions of infestation. Int. J.Psychiatry Med. 27(4): 403-9.Findling, R L, Maxwell, K, Wiznitzer, M. (1997) An open clinical trial of risperidone monotherapy in young children with autistic disorder. Psychopharmacol. Bull.33(1): 155-9Franz, M, Lis, S, Pluddemann, K, Gallhofer, B. (1997) Conventional versus atypical neuroleptics: subjective quality of life in schizophrenic patients. Br J Psychiatry. 170: 422-5Furmaga, KM et al. (1997) Psychosis in medical conditions: response to risperidone. Gen. Hosp. Psychiatry. 19(3): 223-8Ghaemi, S N, Sachs, G S, Baldassano, C F, Truman, C J (1997) Acute treatment of bipolar disorder with adjunctive risperidone in outpatients. Can.J.Psychiatry. 42(2): 196-9.Ghaemi, S N, Sachs, G S.(1997) Long-term risperidone treatment in bipolar disorder: 6-month follow up.Int-Clin-Psychopharmacol. 12(6): 333-8.Gleason, PP, Conigliaro, RL (1997) Neuroleptic malignant syndrome with risperidone. Pharmacotherapy. 17(3): 617-21.Heykants, J et al, (1994) The pharmacokinetics of risperidone in humans: a summary. J.Clin.Psychiatry. 55 Suppl: 13-7.
Huang, ML et al. (1993) Pharmacokinetics of the novel antipsychotic agent risperidone and the prolactin response in healthy subjects. Clin. Pharmacol. Ther. 54(3): 257-68.
Katz IR, Jeste DV, Mintzer JE, et al. (1999) Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry, 60:2, 107-15
Keks, N A (1997) Impact of newer antipsychotics on outcomes in schizophrenia. Clin.Ther. 19(1): 148-58; discussion 126-7.
Kim YK, Kim L, Lee MS. (1999) Risperidone and associated amenorrhea: a report of 5 cases. J Clin Psychiatry, 60:5, 315-7.
Kirov, G K, Murray, R M, Seth, R V, Feeney, S. (1997) Observations on switching patients with schizophrenia to risperidone treatment. Risperidone Switching Study Group. Acta.Psychiatr.Scand. 95(5): 439-43.
Kopala, L C, Good, K P, Honer, W G (1997) Extrapyramidal signs and clinical symptoms in first-episode schizophrenia: response to low-dose risperidone. J. Clin. Psychopharmacol. 17(4): 308-13.
Kopala, L C, Honer, WG. (1997) The use of risperidone in severely demented patients with persistent vocalizations. Int. J. Geriatr. Psychiatry. 12(1): 73-7.
Levin T & Heresco Levy U. (1999) Risperidone-induced rabbit syndrome: an unusual movement disorder caused by an atypical antipsychotic. Eur Neuropsychopharmacol, 9:1-2, 137-9
Luchins, D J, Klass, D, Hanrahan, P, Malan, R, Harris, J (1998) Alteration in the recommended dosing schedule for risperidone. Am. J. Psychiatry. 155(3): 365-6.
Marder, SR, Davis, JM, Chouinard, G. (1997) The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J.Clin.Psychiatry 58(12): 538-46.
Markianos M, Hatzimanolis J, Lykouras L (1999) Gonadal axis hormones in male schizophrenic patients during treatment with haloperidol and after switch to risperidone. Psychopharmacology (Berl), 143:3, 270-2
McDougle, C J et al. (1997) Risperidone treatment of children and adolescents with pervasive developmental disorders: a prospective open-label study. J.Am.Acad.Child Adolesc. Psychiatry. 36(5): 685-93.
Megens, AA (1994) Survey on the pharmacodynamics of the new antipsychotic risperidone. Psychopharmacology Berl. 114(1): 9-23.Muller et al. (1998) Risperidone versus haloperidol and amitriptyline in the treatment of patients with a combined psychotic and depressive syndrome. J.Clin.Psychopharmacol. 18(2): 111-20.Nair, NP. (1998) Therapeutic equivalence of risperidone given once daily and twice daily in patients with schizophrenia. The Risperidone Study Group. J.Clin.Psychopharmacol. 18(2): 103-10.
Nyberg, S etal.. (1993) 5-HT2 and D2 dopamine receptor occupancy in the living human brain. A PET study with risperidone. Psychopharmacology Berl. 110(3): 265-72.
Nyberg S; Eriksson B; Oxenstierna G; Halldin C; Farde L (1999) Suggested minimal effective dose of risperidone based on PET-measured D2 and 5-HT2A receptor occupancy in schizophrenic patients. Am J Psychiatry, 156:6, 869-75
Ravin, D S, & Levenson, J W. (1997) Fatal cardiac event following initiation of risperidone therapy. Ann. Pharmacother. 31(7-8): 867-70.
Saxena, S, Wang, D, Bystritsky, A, Baxter, L R (1996) Risperidone augmentation of SRI treatment for refractory obsessive-compulsive disorder. J. Clin. Psychiatry. 57(7): 303-6.Schotte, A, Janssen, PFM, Gommeren W. (1996) Risperdine compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychpharmacol 124: 57-73.Silberbauer, C. (1998) Risperidone-induced tardive dyskinesia. Pharmacopsychiatry. 31(2): 68-9.Simpson, G M, Lindenmayer, J P. (1997) Extrapyramidal symptoms in patients treated with risperidone. J.Clin.Psychopharmacol. 17(3): 194-201.Singer, S, Richards, C, Boland, RJ. (1995) Two cases of risperidone-induced neuroleptic malignant syndrome [letter]. Am. J.Psychiatry. 152(8): 1234.Singh, AN, Golledge, H, Catalan, J. (1997) Treatment of HIV-related psychotic disorders with risperidone: a series of 21 cases. J.Psychosom.Res. 42(5): 489-93.Sipahimalani, A, & Masand, P S. (1997) Use of risperidone in delirium: case reports.Ann-Clin-Psychiatry. 9(2): 105-7.Song, F. (1997) Risperidone in the treatment of schizophrenia: a meta-analysis of randomized controlled trials.J. Psychopharmacol. Oxf. 11(1): 65-71.Springfield, A.C. Bodiford, E. (1996) An overdose of risperidone. J.Anal.Toxicol. 20(3): 202-3.Stein, DJ, Bouwer, C, Hawkridge, S, Emsley, R A. (1997) Risperidone augmentation of serotonin reuptake inhibitors in obsessive-compulsive and related disorders. J.Clin.Psychiatry. 58(3): 119-22.Still, DJ, et al, (1996) Effects of switching inpatients with treatment-resistant schizophrenia from clozapine to risperidone. Psychiatr. Serv. 47(12): 1382-4.Tekell, JL, Smith, EA, Silva, JA. (1995) Prolonged erection associated with risperidone treatment [letter]. Am. J. Psychiatry. 152(7): 1097.Tooley, P J H & Zuiderwijk, P. (1997) Drug safety: experience with risperidone. Advances in Therapy, 14, 5, 262-266.Tran, P V et al. (1997) Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J. Clin. Psychopharmacol. 17(5): 407-18.Umbricht, D, Kane, JM. (1996) Medical complications of new antipsychotic drugs. Schizophr. Bull. 22(3): 475-83
Viale, G. et al (1997) Impact of risperidone on the use of mental health care resources. Psychiatr. Serv. 48(9): 1153-9.
Wirshing, D A, Wirshing, W C, Kysar, L et al. (1999) Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry, 1999 Jun, 60:6, 358-63
Revised edition of Focus on Risperidone published March 2007
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


