Browse through our Journals...
FORMULATION AND CHARACTERIZATION OF PRAMIPEXOLE LOADED MICROSPHERES
Wakode RR, Bajaj AN
C.U.Shah College of Pharmacy, S.N.D.T Women’s University, Mumbai- 400 049, India
SUMMARY:
The present study involves preparation and evaluation of pramipexole-loaded microspheres. The microspheres were prepared by solvent evaporation method using polymers like ethyl cellulose and hydroxypropylmethyl cellulose. The process parameters were optimized using 23 factorial design. The size, shape and surface morphology of the microspheres were characterized by image analyzer and scanning electron microscopy respectively. In vitro drug release studies were performed and drug release kinectics was evaluated using the linear regression method. Effects of various formulation parameters like stirring rate and concentration of polymer on size and in vitro drug release of microspheres were also studied. The prepared microspheres exhibited prolonged release of pramipexole for a period of 24 hrs. The drug release was found to decrease with increase in polymer concentration. As the stirring speed was increased the size of microspheres were found to decrease, but there was not any significant difference in drug release. The in vitro release kinetics showed that drug release from microspheres is diffusion controlled.
Keywords: pramipexole, solvent evaporation, ethyl cellulose microspheres.
INTRODUCTION
Pramipexole is a non-ergot dopamine agonist recently approved for the treatment of early and advanced Parkinson's disease (PD). It has preferential affinity for the D (3) dopamine receptors, compared to previous dopamine agonists that have higher affinity for D (2) dopamine receptors1,2.
In Parkinson’s disease, the usual dose of pramipexole is 0.125 three times a day, but it is gradually increased to as high as 1.5 mg three to four times a day in advanced stages of disease. So the research project was undertaken with an objective to develop a formulation that can deliver pramipexole for extended periods of time for once a day administration. This may offer significant patient benefits by reducing the number of daily doses compared to conventional therapies.
Oral multiparticulate systems show several advantages in comparison with single unit dosage forms, such as more predictable gastric emptying, gastric emptying less dependent on the state of nutrition, high degree of dispersion in the digestive tract, lesser
risk of dose dumping and reduced local irritation3. An attempt was made to incorporate pramipexole in microspheres using solvent evaporation method. In this process, the polymer was dissolved in a suitable water immiscible solvent and the drug was dispersed or dissolved in this polymeric solution. The resultant solution or dispersion was then emulsified in an aqueous continuous phase to form discrete droplets. In order for the microspheres to form, the organic solvent must first diffuse into aqueous phase and then evaporates at the water/air interface. As solvent evaporation occurs, the microspheres harden and free flowing microspheres can be obtained after suitable filtration and drying4.
MATERIALS AND METHOD
Pramipexole dihydrochloride monohydrate was obtained as gift sample from Sekhsaria Chemicals Ltd. India. Ethyl cellulose and Hydroxypropylmethyl cellulose was obtained as gift sample from Signet Chemicals Ltd and Colorcon Asia Pvt.Ltd respectively. All other chemicals and reagents used were of analytical grade.
PREPARATION OF MICROSPHERES
Microspheres were prepared by the solvent evaporation technique as employed by
Struebel et al. 5,6. Pramipexole, HPMC and EC were dissolved in a mixture of ethanol and dichloromethane (1:1) at room temperature. This was poured into 250 ml water containing 0.01% Tween 80 maintained at a temperature of 30–40 °C and subsequently stirred at ranging agitation speed for 30 min to allow the volatile solvent to evaporate. The microspheres formed were filtered, washed with water and dried in vacuum.
Experimental Design
A 23 full factorial design with 2 replicates was used in this study. The three independent variables investigated were the concentrations of pramipexole (X1), ethyl cellulose (X2) and hydroxypropylmethylcellulose (X3) at two levels7. The design with composition of variables is presented in Table 1. The effect of the previously mentioned variables was investigated on the responses like particle size; encapsulation efficiency and percentage yield as shown in Table 2.
CHARACTERIZATION OF MICROSPHERES:
Determination of encapsulation efficiency
Microspheres (50 mg) were weighed and washed with 5 ml of distilled water for 3 times and centrifuged at 3500 rpm for 10 min. The supernatants were removed and free pramipexole was assayed at 262 nm using UV-Visible Spectrophotometer (JASCO, Japan). The microspheres were then dissolved in 10 ml dichloromethane and transferred to separating funnel; about 5 ml of distilled water was added. The mixture was shaken for 15 mins. The water layer was separated and analyzed for pramipexole. Each measurement was carried out in triplicate.
Pramipexole is highly water-soluble but insoluble in dichloromethane hence the above method of determining encapsulation efficiency was developed.
The encapsulation efficiency (EE) was calculated as follows:
EE (%) = Cactual /Ctheoretical×100
Where, Cactual = actual amount (mg) of drug contained in microspheres
Ctheoretical = Total amount (mg) of drug used initially.
Percentage yield value of microspheres
The percentage yield values was calculated from the ratio of filtered and dried microsphere amount (A) of each formulation to total solid material amount in
the dispersed phase (B)
The percentage yield value = (A/B) × 100)
Particle size:
The microspheres obtained were analyzed for particle size and shape using image analyzer (Leica, model DMLM MPS30) with image analysis software (Soft Imaging Systems, Germany version 5.2). Images of microspheres and particle size distribution data is directly obtained on the system.
Surface morphology
Pramipexole loaded microspheres were characterized for surface morphology using Scanning Electron Microscopy (JEOL, JSM-6400). The microspheres were first coated with gold film under reduced pressure (0.133 Pa) and placed on sample stub. Microspheres were observed at high (800X) and low (400X) magnification to investigate the surface morphology.
a)

b)
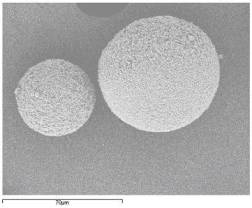
Figure No.1 Scanning Electron Microscopy (batch M6)
- At low magnification 400 X,
- At high magnification 800 X
In vitro release studies
A USP basket apparatus has been used to study in vitro drug release from microspheres. In the present study, drug release was studied using a modified USP XXIV dissolution apparatus type I 8 .Operating temperature was maintained at 37± 0.5oC at 100 rev/min, and the dissolution medium was phosphate buffer pH 7.4, volume being 900 ml. Since the solubility of drug is not pH sensitive, pH change method was not used. Samples of 5 ml were withdrawn at every hour and same amount of dissolution medium was replenished. Samples were filtered and analyzed by HPLC (Tosho, Japan with Ezchrom data management system). The volume was replenished with the same amount of fresh dissolution fluid each time to maintain the sink condition. All experiments were performed in triplicate. Linear regression was used to analyze the in vitro release mechanism.
Statistical analysis
Experimental results were expressed as mean ± SD. Student’s t-test and one-way analysis of variance (ANOVA) were applied to check significant differences in drug release from different formulations. Differences were considered to be statistically significant at p < 0.05. The statistical software used for analysis was Fast Statistics v2.0.4 Build 0627 Demo Version.
RESULT AND DISCUSSION
Pramipexole loaded microspheres were prepared by solvent evaporation using 23 factorial design (Table1).
Table No 1. Independent process parameters and their levels
Sr.no |
Independent process parameters |
Associated variable |
Higher level Code +1 |
Lower level Code -1 |
1 |
Concentration of pramipexole |
X1 |
10 mg/ml |
5 mg/ml |
2 |
Concentration of ethyl cellulose |
X2 |
80 mg/ml |
20 mg/ml |
3 |
Concentration of hydroxypropylmethyl cellulose |
X3 |
40 mg/ml |
10 mg/ml |
It was observed that as the concentration of ethyl cellulose was increased, there was increase in mean particle size of microspheres (p<0.05) Table.2. The viscosity of the medium increases at a higher polymer concentration resulting in enhanced interfacial tension. Shearing efficiency is also diminished at higher viscosities 9,10. This results in the formation of larger particles.
Table No.2 Design of factor combinations and their effects on various responses.
Experiments |
Level of drug Concentration (X1) |
Level of EC concentration (X2) |
Level of HPMC concentration (X3) |
Particle size a (μm) |
EE b (%) |
Yield (%) |
M1 |
+1 |
-1 |
-1 |
350 ± 4.5 |
20 ± 2.6 |
50 |
M2 |
+1 |
+1 |
-1 |
370 ± 3.7 |
33± 2 |
45 |
M3 |
+1 |
-1 |
+1 |
330 ± 6.7 |
41.2± 3.7 |
40 |
M4 |
-1 |
-1 |
+1 |
345 ± 3.9 |
43 ± 3 |
50 |
M5 |
-1 |
+1 |
+1 |
400 ± 5.7 |
52.6 ± 1.9 |
55 |
M6 |
-1 |
+1 |
-1 |
350 ± 4.5 |
67± 2.5 |
85 |
M7 |
-1 |
-1 |
-1 |
315 ± 2.4 |
29 ± 2.7 |
38 |
M8 |
+1 |
+1 |
+1 |
410 ± 6.8 |
55 ± 3.6 |
47 |
X1: Concentration of drug X2: Concentration of ethyl cellulose X3: Concentration of HPMC K4M
Where,
Drug: pramipexole, EC: Ethyl cellulose, HPMC: HPMC K4M,
EE: Encapsulation Efficiency
a Mean ± SD, n = 10
b Mean ± SD, n = 6
In vitro release profiles of the batches prepared using factorial design (Figure.3). It was observed that increasing ethyl cellulose concentration decreased the in vitro release of pramipexole (p<0.05). Increased density of the polymer matrix at higher concentrations results in an increased diffusional pathlength, which resulted in decrease drug release. When concentration of HPMC K4M was used at higher level in the formulation the drug was released at faster rate. This was due to increase in hydrophilicity of the microsphere matrix. From figure3, it was observed that batch M6 tend to give prolonged sustained effect of pramipexole over a period of 24 hrs as compared to other batches. Hence Batch M6 was considered as the optimized batch. To study the effect of agitation different formulations using optimized formula (batch M6) were prepared. It was found that as the speed of agitation was increased to 1200 rpm, the size of microspheres decreased.
Table No.3 In vitro release data fitted into various models.
Batches |
Mathematical model |
|||||||
Higuchi |
Korsmeyer equation |
First order |
Zero order |
|||||
R2 |
r |
R2 |
r |
R2 |
r |
R2 |
r |
|
M1 |
0.9512 |
0.9677 |
0.9234 |
0.9134 |
0.8813 |
0.8879 |
0.8012 |
0.8121 |
M2 |
0.9123 |
0.9345 |
0.9321 |
0.9561 |
0.8654 |
0.8345 |
0.8112 |
0.8210 |
M3 |
0.9524 |
0.9615 |
0.9124 |
0.9415 |
0.8978 |
0.8811 |
0.8143 |
0.8362 |
M4 |
0.9543 |
0.9657 |
0.9556 |
0.9612 |
0.8121 |
0.8231 |
0.8001 |
0.8134 |
M5 |
0.9111 |
0.9321 |
0.9456 |
0.9523 |
0.8334 |
0.8122 |
0.8102 |
0.8245 |
M6 |
0.9831 |
0.9915 |
0.9689 |
0.9625 |
0.8598 |
0.8455 |
0.8123 |
0.8345 |
M7 |
0.9634 |
0.9745 |
0.9556 |
0.9459 |
0.8889 |
0.8772 |
0.8347 |
0.8456 |
M8 |
0.9567 |
0.9687 |
0.9767 |
0.9536 |
0.8754 |
0.8542 |
0.8239 |
0.8532 |
R2 - goodness of fit; r - correlation coefficient
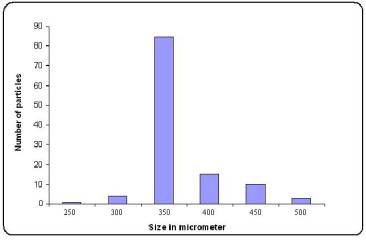
Figure No.2 Particle size distribution of pramipexole-loaded microspheres
(Batch M6)
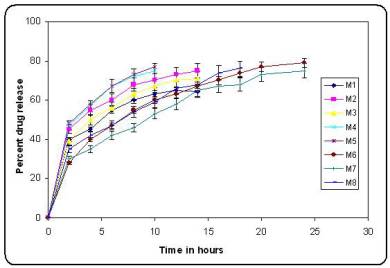
Figure no 3. In vitro release profiles of batches prepared using factorial design
(bars represent mean ± SD; n = 3)
The mean particle size obtained was in range 250-300 mm, but the yield obtained was about 50%, since there was formation of fibres. Further increase in speed caused polymer to precipitate and only fibres were obtained. The optimum speed of agitation was 900 rpm.
The in vitro release of microspheres was fitted into different mathematical model like Higuchi release, Korsmeyer equation, first order release and zero order release (Table 3). The interpretation of data was based on the value of the resulting regression coefficients 11,12. The in vitro drug release showed the highest regression coefficient values for Higuchi’s model, indicating diffusion to be the predominant mechanism of drug release (Figure 4).
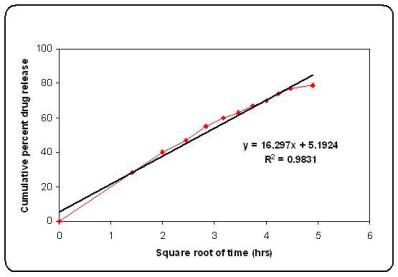
Figure No.4 Higuchi plot for batch M6
Surface morphology showed that microspheres obtained were spherical with smooth surface (Figure1 a,b). The particle size distribution (Figure2) showed that microspheres were in size range of 250-500mm. Almost 85 % of particles obtained were 350 ± 4.5 mm in size.
CONCLUSION
Pramipexole loaded microspheres were successfully prepared by solvent evaporation method. Varying concentration of polymer high entrapment efficiency of about 67 % was achieved for highly water-soluble drug like pramipexole. The microspheres tend to give prolonged release of pramipexole over a period of 24 hrs. The drug release was found to be diffusion controlled.
ACKNOWLEDGEMENTS
The authors are thankful to Sekhsaria Chemicals Ltd. and Colorcon Asia Pvt.Ltd, India for providing support.
References
1. Imamura A. Uitti R , et.al, Dopamine agonist therapy for Parkinson Disease and
pathological gambling, Parkinsonism and Related Disorders, short communication,
2006,12,506-508.
2. Benbir G., Ozekmeksi S, et.al., Risk factors in development of motor complications in
555 Pakinson’s patients on levodopa therapy, Clinical Neur.Neurosurgery; 2006,108,
726-732.
3. Hagalavadi S, Patel B; Design and statistical optimization of glipizide loaded
lipospheres using response surface methodology; Acta Pharm. 2007, 57, 269–285.
4. Donnell P, James W, Preparation of microspheres by the solvent evaporation
technique, Advanced Drug Delivery Reviews, 1997, 28, 25–42.
5. Kumar A, Ridhurkar D, et.al; Floating microspheres of cimetidine: Formulation,
characterization and in vitro evaluation; Acta Pharm. 2005, 55,277-285.
6. Struebel A, Siepmann J and Bodmeier R; Multiple units gastroretentive drug
delivery systems: a new preparation method for low density microspheres, J.
Microencaps.2003, 20, 329–347.
7. Dubey R, Parikh R; Factorial Effect of Process Parameters on Pharmaceutical
Characteristics & Stability Study of PLGA Microspheres Containing Water-Soluble
Drug; Drug delivery technology 2004, 4.
8. The United States Pharmacopoeia XXIV, United States Pharmacopoeial Convention,
Rockville 2000,1941–1943.
9. Reddy B. P., Dorle A. K.and Krishna D. K; Albumin microspheres: effect of process
variables on the distribution and in vitro release, Drug Dev. Ind. Pharm. 1990, 16
1781–1803.
10. Ishizaka T., Preparation of egg albumin microspheres and microcapsules, J. Pharm. Sci. 1981, 70, 358–361.
11. Wagner J. G., Interpretation of percent dissolved-time plots derived from in vitro
testing of conventional tablets and capsules, J. Pharm. Sci. 1969, 58,1253–1257.
12. Schefter E.and Higuchi T., Dissolution behavior of crystalline solvated and non-
solvated forms of some pharmaceuticals, J. Pharm. Sci. 1963, 52781–791.
FIRST PUBLISHED FEBRUARY 2008
COPYRIGHT PRIORY LODGE EDUCATION LIMITED
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


