Browse through our Medical Journals...
Reversible posterior leucoencephalopathy syndrome in a postpartum patient
DR SK VIJAYAN, (Anaesthetics and ITU)
WESTON GENERAL HOSPITAL
Abstract
This is a case of a 30 year old primiparous mother who developed hypertension with a severe headache in the immediate postpartum period. She then suffered a generalised tonic clonic seizure on the 10th postpartum day. Apart from her symptoms of severe headache and convulsion, she had no symptoms and no proteinuria or other biochemical or haematological changes associated with eclampsia The CT and MRI findings were consistent with vasogenic oedema in the right posterior parieto occipital white matter and these are consistent with reversible leucoencephalopathic syndrome. The differential diagnosis of convulsions in the immediate postpartum period is discussed and radiological features of reversible leucoencephalopathy syndrome are described.
Introduction
Reversible posterior leucoencephalopathy syndrome was first described in 1996 by Hinchey et al.1 The presenting features are headache, vomiting, confusion, seizures, visual abnormalities and motor signs. Reversible posterior leucoencephalopathy syndrome is associated with a variety of underlying conditions including hypertensive encephalopathy, preeclampsia, systemic lupus erythematosus, thrombotic thrombocytopenia purpura, treatment with immunosuppressants, renal failure and central nervous system infections.
Bilateral white matter abnormalities suggestive of oedema are seen on computed tomography (CT) or magnetic resonance imaging (MRI) scanning in the posterior parieto-occipital regions of the cerebral hemispheres. Importantly, these changes appear to be completely reversible if the underlying cause is treated or the precipitating drug withdrawn (or the dose reduced) early in the clinical course.
This is a case report of reversible posterior leucoencephalopathy syndrome in a postpartum patient.
Case report
The patient was a 30-year-old primip with a BMI of 21.8 kg/m2 at booking and had no pre-existing medical problems. She reported headache on several occasions. She was taking paracetamol 1 g and codeine phosphate 60 mg as required for pain and codeine linctus to ease her cough. She delivered a live female infant by LSCS at 39 weeks. On arrival in theatre it was noted that her blood pressure was 140/70 mmHg (booking blood pressure 100/60 mmHg.). Following a 500-mL crystalloid preload, spinal anaesthesia was given. Two millilitres of hyperbaric 0.5% bupivacaine and diamorphine 300 μg were administered as a single shot using a 25-gauge pencil-point needle. This provided an adequate block for surgery. During the procedure her blood pressure remained at between 140 and 152 mmHg systolic and the pulse rate 60 to 65 beats/min. Diclofenac 75 mg and paracetamol 1 g were given rectally at the end of the procedure.
In the post-anaesthesia care unit her blood pressure increased to 180/95 mmHg over the next hour accompanied by worsening headache. It was thought that the hypertension might be the result of pain, therefore codeine phosphate 60 mg was given orally. After this both the blood pressure (150/75 mmHg) and headache improved. The blood pressure was thereafter monitored regularly as there was a concern that our patient was developing preeclampsia. However, the blood pressure settled without further treatment and she had no proteinuria. She was discharged on day three post partum with a blood pressure of 109/66 mmHg and no headache.
At home she developed headache again and this time headache was generalised but the pain was worse in the neck and occiput. On the tenth pos-partum day she presented to emergency department with headache and she suffered a witnessed generalised tonic-clonic seizure. No proteinuria was detected. Her blood results were essentilally normal with normal white cell count and biochemistry. A CT scan of the brain showed an area of low attenuation in the right occiput. She underwent lumbar puncture, which showed a normal opening pressure. On light microscopy no organisms were seen. The white blood cell and red blood cell counts were both <1 × 106/L. Culture showed no growth after 40 h. The CSF protein concentration was 0.3 g/L and glucose 2.9 mmol/L.
MRI scan of the brain showed changes consistent with reversible posterior leucoencephalopathy syndrome. She was discharged on regular analgesics. The blood pressure was monitored regularly by her general practitioner and the medications were decreased over the course of the next month and then stopped.
Discussion
We present a case of a post-partum patient who had a generalised tonic-clonic seizure associated with changes in the occipital lobe shown by both CT and MRI imaging. These symptoms and scan changes resolved completely within three months on oral antihypertensive treatment.
The differential diagnosis for seizures in the early post-partum period includes eclampsia, subarachnoid haemorrhage, intracerebral haemorrhage, thrombotic phenomena, intracranial neoplasm, head trauma, idiopathic epilepsy, infection (meningo-encephalitis), amniotic fluid embolism, post-partum angiopathy (see below) and reversible posterior leucoencephalopathy syndrome.
Focal neurology and changes on imaging are unusual in eclampsia. Although our patient was hypertensive, she demonstrated no other stigmata of eclampsia. However, it has been suggested that reversible posterior leucoencephalopathy syndrome could be considered to be an indicator of preeclampsia even if other features are absent.5 Her blood pressure (maximum 180/95 mmHg five days before her seizure while in the PACU) was lower than that usually required to precipitate reversible posterior leucoencephalopathy syndrome if it is due to hypertension, although it is possible that fluid shifts during delivery and the puerperium could have resulted in a lower threshold. Typically for hypertensive encephalopathy the blood pressure will approach or even exceed 250/150 mmHg, although lower pressures have been reported in both chronic renal failure (200/110 mmHg) and preeclampsia (183/96 mmHg).[2], [6], [7], [8], [9] and [10]
Haemorrhage was an unlikely cause of seizures in this patient as imaging showed no signs of intracranial haemorrhage. Imaging did not reveal evidence of ischaemia secondary to thromboembolism or vasospasm or of space occupying lesion. There was no history of head injury or pre-existing epilepsy. Infection was a possibility as the dura had been breached with the spinal block. The systemic white blood cell count was 8.4 × 109/L. Amniotic fluid embolism rarely occurs after 48 h post partum and generally presents with cardiopulmonary collapse and coagulopathy.
Reversible posterior leucoencephalopathy syndrome was thought to be the most likely diagnosis in this patient. She had headache, focal visual abnormalities and a seizure. MRI findings in this syndrome typically are consistent with vasogenic oedema. This is most marked in the posterior parieto-occipital white matter and corticomedullary junction but may also involve the cerebral cortex, brainstem, basal ganglia, frontal lobes or cerebellum. It has been noted that fluid-attenuated inversion recovery imaging was particularly useful in demonstrating the posterior leucoencephalopathy syndrome lesion.[2] and [3] Use of diffusion-weighted imaging and apparent diffusion coefficient maps allow differentiation between vasogenic oedema and infarction.4 CT scanning can also be used and shows the lesions satisfactorily. Her CT and MRI appearances were consistent with those seen in reversible posterior leucoencephalopathy syndrome and resolved completely on antihypertensive treatment. Reversible posterior leucoencephalopathy syndrome is still under-recognised and therefore under-treated. If treatment is delayed then irreversible changes can occur. The incidence in the peripartum setting is not known.
The exact cause of reversible posterior leucoencephalopathy syndrome remains unclear. It is likely that it results from a rise in blood pressure that overcomes the autoregulatory capacity of cerebral blood flow. This causes dilatation of cerebral arterioles and results in leakage though endothelial tight junctions. If the endothelial barrier is damaged then this can occur at lower pressures. An alternative hypothesis is that patients develop vasospasm secondary to hypertension. However, if this were the cause of the symptoms, MRI would be expected to show ischaemia either instead of, or in addition to, oedema. The mainstay of treatment of reversible posterior leucoencephalopathy syndrome is recognition and removal of the precipitating factor. The majority of patients in the puerperium who present with this syndrome will have preeclampsia and therefore efforts will be appropriately concentrated on controlling the blood pressure. It is therefore likely that our current management of these patients prevents long-term neurological damage. Reversible posterior leucoencephalopathy syndrome is also seen in renal failure, immunosuppression and secondary to chemotherapy.
An alternative explanation is that our patient had post-partum angiopathy. This diagnosis should be considered in a post-partum patient with hypertension and headache but no proteinuria. Post-partum angiopathy is a form of reversible cerebral segmental vasoconstriction characterised by severe ‘thunderclap’ headaches, seizures, focal neurological deficits and segmental narrowing and dilation of large and medium sized arteries. Typically scanning reveals ischaemic lesions but MRI findings consistent with reversible posterior leucoencephalopathy syndrome have been reported.[11] and [12]
In summary, reversible posterior leucoencephalopathy syndrome should be considered in patients presenting with headache, vomiting, confusion, seizures or focal visual or motor signs in the presence of a precipitating factor. In patients presenting in the peripartum period it represents a differential diagnosis to preeclampsia but preeclampsia is also a precipitating factor. Management consists of careful control of blood pressure. The posterior reversible leukoencephalopathy syndrome (PRES) is a recently proposed cliniconeuroradiologic entity.The most common causes of PRES are hypertensive encephalopathy, eclampsia, cyclosporin A neurotoxicity and the uremic encephalopathies.Most patients are markedly hypertensive at presentation, although some have only mildly elevated or even normal blood pressure. Symptoms may include headache, nausea, vomiting, altered mental status, seizures,stupor, and visual disturbances. On CT and MR studies, edema has been reported in a relatively symmetrical pattern, typically in the subcortical white matter and occasionally in the cortex of the occipital and parietal lobes. These often striking imaging findings usually are resolved on follow-up studies obtained after appropriate therapy. Diffusion-weighted images would not show hyperintense signal because of the presence of interstitial rather than cytotoxic edema. We report a case of PRES due to hypertensive encephalopathy studied by CT and MRI.
Apparent diffusion coefficient map was useful for distinction of this vasogenic edema from cytotoxic edema due to brain infarction. Under the diagnosis of RPLS, we successfully treated her disease using a trinitroglycerin as an antihypertensive, a hyperosmolar agent, methylprednisolone, and a free radical scavenger. Postpartum women may have the risk of development of PRES even without preeclampsia-eclampsia. Vascular endothelial dysfunction may trigger PRES, in addition to acute and modest increase in systemic pressure.
The hallmark of this entity is reversible parieto-occipital white matter edema as seen on magnetic resonance imaging (MRI). Advanced MRI techniques, such as echo-planar diffusion-weighted images and apparent diffusion coefficient maps, suggest cerebral artery dilatation as the underlying mechanism. Laboratory findings and computed tomography (CT) scans are typically unremarkable. PRES has a favorable prognosis if treated promptly and appropriately.
Literature Review
Reversible Posterior Leukoencephalopathy Syndrome was introduced into clinical practice in 1996 in order to describe unique syndrome, clinically expressed during hypertensive and uremic encephalopathy, eclampsia and during immunosuppressive therapy [1]. First clinical investigations showed that leucoencephalopathy is major characteristic of the syndrome, but further investigations showed no significant destruction in white cerebral tissue [13]. In majority of cases changes are localise in posterior irrigation area of the brain and in the most severe cases anterior region is also involved. Taking into consideration all above mentioned facts, the suggested term was Posterior Reversible Encephalopathy Syndrome (PRES) for the syndrome clinically expressed by neurological manifestations derived from cortical and subcortical changes localised in posterior regions of cerebral hemispheres, cerebral trunk and cerebellum [5].
CLINICAL PICTURE:
Most common symptoms are headache, nausea, vomiting, confusion, behavioural changes, changes of conciousness (from somnolencia to stupor), vision disturbances (blurred vision, haemianopsia, cortical blindness) and epileptic manifestations (mostly focal attacks with secondary generalisation). Mental functions are characterised with decreased activity and reactivity, confusion, loss of concentration and mild type of amnesia. Lethargy is often initial sign, sometimes accompanied with phases of agitation. Stupor and coma rarely occurred.
PRES is a clinicoradiological syndrome associated with the following clinical
• Hypertensive encephalopathy
• Preclampsia/eclampsia/HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome
• Immunosuppressive/cytotoxic drugs (e.g., cyclosporin, antineoplastic drugs, interferon-α, antiretroviral therapy)
• Acute or chronic renal diseases (e.g., glomerulonephritis, dialysis disequilibrium syndrome)
• Thrombotic thrombocytopenic purpura/haemolytic uraemic syndrome
• High-dose steroid therapy
• Liver failure (e.g., drug-induced)/transplantation
• Endocrine dysfunctions (e.g., primary aldosteronism, pheochromocitoma)
• Hypercalcemia/hyperparathyroidism
• Bone marrow transplantation
• Massive blood transfusion/erythropoietin therapy
• Porphyria
• Other causes (e.g., intravenous globulin, contrast media exposure, scorpion poison, stimulants abuse, digitoxin intoxication, Averrhoa carambola ingestion)
The clinical picture is characterized by an acute encephalopathywith headache, vomiting, confusion, seizures, and visual impairment. Patients have an acute or subacute neurological presentation, often heralded by convulsions. Seizures may begin focally but usually become generalized. Multiple seizures are more common than single events, sometimes presenting a true status epilepticus. Alertness and behavior disturbancesrange from somnolence and lethargy to stupor and frankcoma; restlessness and agitation may alternate with lethargy. Visual disturbances are almost always presentranging from hemianopsia and visual neglect to cortical blindness. Tendon reflexes are usually brisk and some patients may also present weakness and incoordination of the limbs
Pathophysiology and Pathological features
The exact pathogenesis of PRES remains incompletely understood but is probably related to the failure cerebral autoregulation and endothelial damage. The favored pathogenetic theory suggests autoregulatory disturbance with hyperperfusion, resulting in blood-brain barrier breakdown with reversible edema, without infarction. In particular, in conditions accompanied by high blood pressure (e.g., hypertensive encephalopathy) it has been suggested that the increased systemic pressure exceeds the autoregulatory mechanisms of the cerebral vasculature, sufficient to overcome the blood-brain barrier, and hence allowing extravasation of fluid and blood into the brain parenchyma [4] . Thus there is a “breakthrough” of auto regulation and increasing blood pressure, an upper limit of autoregulation is surpassed, causing focal dilatation of cerebral vessels. Regions of both vasoconstriction and vasodilatation (“sausage-string pattern”) develop [17], especially in arterial boundary zones, resulting in brain hyperperfusion. This leads to focal areas of breakdown of the blood-brain barrier and extravasation of fluid.
Autopsy studies in patients with hypertensive encephalopathy or eclampsia show varying degrees of vascular (fibrinoid necrosis and of thrombosis arterioles and capillaries) and parenchymal (microinfarcts, cerebral edema) alterations. However, brain biopsy findings have been reported that show edematous white matter with no evidence of vessel wall damage or infarction, thus supporting the concept that the imaging changes on MRI represent vasogenic edema. It is not well known why the posterior circulation is preferentially affected. A possible explanation is the lower sympathetic innervation of posterior cerebral arterial circulation than in the internal carotid artery territory, with a consequent reduced autoregulation of already impaired cerebral areas.
An alternative theory for PRES suggests spasm of cerebral arteries in response to acute hypertension, resulting in decreased cerebral blood flow and ischemia and cytotoxic edema, especially in the border zones between arterial territories (“watershed zones”). Finally, cytotoxic therapies may have direct toxicity on the vascular endothelium, leading to brain capillary leakage, and blood-brain barrier disruption which triggers vasogenic edema [1]. However, further research is needed for a better comprehension of pathophysiology of this complex condition.
DIAGNOSIS:
In patients with hypertensive encephalopathy and eclampsia high blod pressure is registered. Neurological examination revealed vision changes and damages of mental function as well as increased reflex activity. Today, brain MRI and CT are considered the most important diagnostic method for the diagnosis and follow-up of patients with PRES [6]. Brain MRI better detects smaller focal parenhim abnormalities than brain CT. The most often neuroradiological finding is relatively symmetrical oedema of white cerebral tissue in parieto-occipital regions of both cerebral hemispheres. Gray cerebral tissue is sometimes involved, usually in mild form of disease. Diagnosis of this "cortical" form of PRES is possible by MR FLAIR (Fluid-Attenuated Inversion Recovery) technique [5].
Radiographic findings
MRI Findings
Eclampsia is one of the main causes of Posterior Reversible Encephalopathy Syndrome (PRES) a recent clinico-neuroradiological entity represented by characteristic MR findings of a symmetric bilateral subcortical/cortical hyperintensity in T2-weighted images, more often in parieto-occipital lobes, accompanied by clinical neurological alterations. Neuroradiological and clinical alterations are commonly completely reversible although ischemic evolution has been described. The pathophysiology is still a matter of debate. Specific magnetic resonance (MR) techniques, such as FLAIR (fluid attenuated inversion recovery) and DWI (diffusion weighted images) sequences, have improved the ability to detect subcortical/cortical lesions and helped to clarify the underlying pathophysiological mechanism of cerebrovascular involvement, which results important for an appropriate therapeutic decision. Recognition of the characteristic imaging findings by radiologists is the key to diagnosing this syndrome and preventing deleterious workups or therapies.
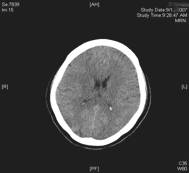
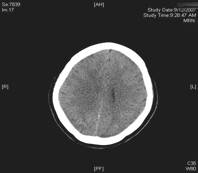
Fig. 1 Noncontrast computed tomography showing diffuse hypodensity in posterior regions bilaterally
CT can be used preliminarily to detect hypodencelesions of posterior encephalopathy, but magnetic resonance imaging (MRI) is the gold standard [1, 2, 3, 7]. Due to the widespread use of MRI this syndrome is being diagnosed more frequently. During the acute phase MRI usually reveals hyperintence on both echoes of a dual-echo T2-weighted sequences and either iso- or hypointence on T1-weighted images brain abnormalities, including both gray and white matter. Mainly involved are the parietal-occipital lobes; however, cerebellar hemispheres, basal ganglia, frontal lobes, and brainstem are also often involved [1, 13]. Fluid-attenuated inversion recovery sequences significantly improve the ability to diagnose and detect subcortical and cortical lesions in PRES over proton density and T2-weighted spin-echo images and therefore should be performed in patients with suspected PRES to allow more confident recognition of the often subtle imaging abnormalities[13]. Diffusion weighted MRI has been demonstrated to be particularly sensitive to changes in distribution of water in the brain and can detect white matter edema early and reliably differentiate between vasogenic and cytotoxic edema in these patients (Fig. 1) [7, 8].
A spectrum of severity has been hypothesized, ranging from an initially reversible phase of vasogenic edema formation to a later phase of ischemic damage and hemorrhage, which carries a worse prognosis [9]. Magnetic resonance spectroscopy has been also suggested having a prognostic value during the acute phase, when high lactate levels may be present probably due to transient derangement of energy metabolism [3, 4]. Finally, the use of perfusion computed tomography with stable xenon, a technique providing a quantitative measure of cerebral perfusion, has been recently proposed in the early stage to monitor the degree of cerebral perfusion and to guide titration of antihypertensive therapy.
Outcome
One of the distinctive characteristics of PRES is the reversibility of the clinical and radiological abnormalities once treatment is instituted [1, 3, 6]. Most patients usually make a complete recovery within few weeks [1]. It can also be thought that despite the benign course during the acute phase some patients may later develop chronic neurological sequaele, for example, focal epilepsy. Cases of hypertensive encephalopathy or eclampsia followed by later development of chronic epilepsy have been reported in the literature. Recurrent seizures starting from weeks to years after the acute encephalopathy and neuroradiological study reveal lateralized hypocampal sclerosis or posterior brain lesions. In these patients posterior encephalopathy acted as a precipitating injury leading to a permanent cerebral damage and possibly triggering a chronic epileptic focus.
MRI findings suggest the development of the transient, “edema” lesions into malacic areas. However, as acute neuroimaging study is not available for any of these cases, this mechanism remains speculative.
Differential Diagnosis
The differential diagnosis of PRES covers a wide spectrum of neurological and extraneurological diseases:
• CNS vasculitis
– Granulomatous angiitis
– Systemic lupus erythematosus
– Polyarteritis nodosa
• Acute or subacute neurological diseases
– Ischemic stroke
– Progressive multifocal leukoencephalopathy
– Acute disseminated encephalomyelitis
– Infective encephalitis
– Cerebral venous thrombosis
– Cerebral autosomal dominant arteriopathy with stroke and ischemic leukoencephalopathy (CADASIL)
• Mithocondrial diseases
– Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS)
– Myoclonic epilepsy and ragged red fibers syndrome (MERRF)
• CNS collagen diseases
• Hypoglycemia/hyponatremia
Principal differential diagnoses include sinus/cerebral vein thrombosis and acute cerebral ischemia, which are ruled out by MRI examinations. Infectious/autoimmuneinflammatory disorders are usually excluded because of the clinical course, negative findings on multiple blood and CSF cultures, no significant increase in CSF white blood cells, and negative serological studies. Moreover, the distinction between PRES and extensive ischemic stroke has very relevant therapeutic as well as prognostic implications for the approach to controlling the hypertension and the possible reversibility of neurological deficit in PRES. Generally, special investigations (e.g., CSF examination) are not necessary except from a clinical suspect of infectious/autoimmune-inflammatory disorders (e.g., primary angiitis of brain) in which a reactive CSF picture is observed.
Stepwise treatment of PRES is as follows:
• Removal/significant reduction in the causative factors/ medications
• Maintenance of hydration (intravenous crystalloid fluids), adequate arterial oxygenation, correction of electrolyte disturbances
• Monitoring of airways and ventilation. Intubation (if insufficient oxygenation)
• Insertion of central venous catheter (if cardiac dysfunction)
• Delivery or cesarean section in pregnant women
• Lowering of blood pressure (mean arterial blood pressure: 105–125 mmHg with not more that 25% reduction within the first hour)
Intravenous therapy preferred (first agents: nicardipine at 5–15 mg/h, labetalol at 2–3 mg/min, or nimodipine; second-line agents: sodium nitroprussiate, hydralazine, and diazoxide).
Treatment of hypertension
Correction of systemic blood pressure abnormalities is crucial since, if untreated, it may lead to development or exacerbation of cerebral edema. On the other hand, too rapid correction of systemic hypertension in pregnant women may result in compromised uteroplacental blood flow. In general, the goal of treatment should be to reduce mean blood pressure to approximately premorbid levels; it should be monitored with a radial arterial catheter and maintained between 105–125 mmHg, preferably by intravenous antihypertensive medications. Nicardipine (5–15 mg/h) or labetalol (2–3 mg/min) are usual first-line agents. Parenterally administrated nimodipine, a cerebral selective calcium-channel blocker, may be useful in preventing cerebral vasospasm, a pathogenetic mechanisms demonstrated in pregnant patients with hypertensive leukoencephalopathy [14]. In addition, a putative neuroprotective effect of nimodipine has been also suggested [14]. Several clinical studies have also shown that intravascular magnesium sulphate safely relieved maternal cerebral vasospasm [15].
Conclusions
Although it is a well known condition among neuroradiologists, PRES is still unfamiliar to many clinicians working in critical areas. Delay in diagnosis of this condition may lead to additional morbidity and prolonged ICU stay. Thus it is very important that intensivists and all physicians be well aware of this syndrome since prompt recognition and precocious treatment have prognostic implications.
References
1. Hinchey J, Chaves C, Appignani B,Breen J, Pao L, Wang A, Pessin M,Lamy C, Mas JLM, Caplan LR (1996) A reversible posterior encephalopathy syndrome. N Engl J Med 334:494–500.
2. R. Bakshi, V.E. Bates, L.L. Mechtler, P.R. Kinkel and W.R. Kinkel, Occipital lobe seizures as the major clinical manifestation of reversible posterior leukoencephalopathy syndrome: magnetic resonance imaging findings, Epilepsia 39 (1998), pp. 295–299.
3. A.B. Singhal, Postpartum angiopathy with reversible posterior leukoencephalopathy, Arch Neurol 61 (2004), pp. 411–416.
4. H. Ay, F.S. Buonanno and P.W. Schaefer et al., Posterior leukoencephalopathy without severe hypertension: utility of diffusion weighted MRI, Neurology 51 (1998), pp. 1369–1376.
5. E.C. Raps, S.L. Galetta and M. Broderick et al., Delayed peripartum vasculopathy: cerebral eclampsia revisited, Ann Neurol 33 (1993), pp. 222–225.
6. E.M. Chester, D.P. Agamanolis, B.Q. Banker and M. Victor, Hypertensive encepalopathy: a clinicopathologic study of 20 cases, Neurology 28 (1978), pp. 928–939.
7. S.J. Phillips and J.P. Whisnant, Hypertension and the brain, Arch Intern Med 152 (1992), pp. 938–945.
8. E.B. Healton, J.C. Brust, D.A. Fienfeld and G.E. Thomson, Hypertensive encephalopathy and the neurologic manifestations of malignant hypertension, Neurology 32 (1982), pp. 127–132.
9. R.W. Gifford, Management of hypertensive crises, JAMA 266 (1991), pp. 829–835.
10. Soltes, I.M. Schmalfuss and M.T. Bhatti, Cortical blindness due to reversible posterior leukoencephalopathy syndrome in a patient with thrombotic thrombocytopenic purpura and preeclampsia, Arch Ophthalmol 122 (2004), pp. 1885–1887.
11. R.K. Garg, Posterior leukoencephalopathy syndrome, Postgrad Med J 77 (2001), pp. 24–28.
12. D.W. Dodick, Reversible segmental cerebral vasoconstriction (Call-Fleming syndrome): the role of calcium antagonists, Cephalalgia 23 (2003), pp. 163–165
13. Casey SO, Sampaio RC, Michel E, Truwit CL (2000) Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol 21:1199–1206.
14. Scmid-Elaesser R, Kunz M, Zausinger S, Prueckner S, BRiegel J, Steiger HJ. Intravenous magnesium versus nimodipine in the treatment of patients with anesurysmal subarachnoid haemorrhage: a randomized study. Neurosurgey 2006; 1054-65.
15. Belfort MA, Moise KJ Jr. Effect of magnesium sulphate on maternal brain blood flow in preeclampsia: a randomised placebo-controlled study. Am J obstet Gynaecol 1992;167:661-6
16. Weidaauer S, Gaa J, Sitzer M, Hefner R, Lanfermann H, Zanella FE(20030 Posterior encephalopathy with vasospasm: MRI and angiography.
17. Strangaard S, Jones JV, Mackenzie ET, Graham DI, Farrar JK (1976) The sausage-string pattern in the pial vessels in acute, angiotensin-induced hypertension-vasospasm or vasodilatation? Acta Med Scand Suppl 602:9-12
First Published December 2007
Copyright Priory Lodge Education Limited 2007
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


