Browse through our Journals...
Antibacterial activity of calcium hydroxide and chlorhexidine against Enterococcus faecalis in human root canal ex vivo
María Gabriela PACIOS, DDS1, Clara SILVA, DBC, PhD2, María Elena LÓPEZ*, DBC, PhD1, Marta CECILIA, DBC, PhD2
1 Cátedra de Química Biológica, Facultad de Odontología, Universidad Nacional de Tucumán
2 Cátedra de Bacteriología, Instituto de Microbiología “Luis C. Verna”, Facultad de Bioquímica, Química y Farmacia, Universidad Nacional de Tucumán
Corresponding author: Dra. María Elena López
Cátedra de Química Biológica
Facultad de Odontología–UNT
Av. Benjamín Aráoz 800
(4000) – San Miguel de Tucumán - Argentina
Fax N°: 54-381-4227589
Telephone N°: 54-381-4107317
E-mail: mariae.lopez@odontologia.unt.edu.ar
Home Telephone N°: 54-381-4239564
Summary
Enterococcus faecalis is a persistent agent that frequently causes infection of the tooth root canal and failure of endodontic treatments.
The aim of this work was to evaluate the antimicrobial activity of Ca(OH)2, chlorhexidine and Ca(OH)2 + chlorhexidine in root canals infected with Enterococcus faecalis.
Maxillary anterior human teeth were prepared, sterilized and infected with Enterococcus faecalis for 3 days. Specimens were filled with Ca(OH)2 + distilled water, 2% chlorhexidine gel or Ca(OH)2 + 2% chlorhexidine and incubated at 37ºC. At different times the dressings were removed and the teeth were immersed in Brain Heart Infusion broth. Enterococcus faecalis growth was evaluated by monitoring turbidity of the culture medium. Specimens were observed by scanning electron microscopy.
Chlorhexidine gel was effective in eliminating Enterococcus faecalis at day 1. Ca(OH)2 + distilled water and Ca(OH)2 + chlorhexidine showed no antimicrobial effect on Enterococcus faecalis. Scanning electron microscopy observations evidenced these results.
Chlorhexidine gel was the only effective intracanal medicament against Enterococcus faecalis.
Keywords: Ca(OH)2. Chlorhexidine. Enterococcus faecalis. Root canal
INTRODUCTION
The success of root canal treatment is dependent on mechanical preparation, irrigation, microbial control, and complete obturation of the root canal. However, the occurrence of persistent root canal infection is a common clinical problem in endodontics.
Chemomechanical preparation cannot eliminate all the bacteria from the root canal system. Byström et al (1985), demonstrated that instrumentation and irrigation will eliminate bacteria in only approximately 50% of root canals. The remaining bacteria may multiply during the period between appointments, in cases where the canal is not dressed with a disinfectant. However, when biomechanical preparation is combined with placement of an antimicrobial dressing before root canal obturation, bacteria can be more effectively eliminated (Sjögren et al, 1991).
Dentinal tubules may act as an important reservoir for bacteria that can lead to re-infection of the root canal and ultimately the failure of endodontic treatment (Love, 2001). Numerous studies have shown that persistent endodontic infections are often caused by Enterococcus faecalis. Molander et al (1998), examined the microbiological status of the roots of filled teeth with periradicular lesions and found E. faecalis in 32% of the investigated teeth. Peciuliene et al (2000), found E. faecalis in 71% of culture-positive teeth with apical periodontitis requiring retreatment. E. faecalis seems to be highly resistant to the medications used during treatment. This bacteria is easily destroyed when grown in vitro (Almyroudi et al, 2002; Gomes et al, 2001) but becomes resistant in the environment of the root canal system. There are several virulence factors which makes E. faecalis more resistant. It may undergo changes in the root canal system, possibly activating a virulence factors including adherence to host cells, expression of proteins to ensure cell survival as a result of altered environmental nutrient supply, the ability to compete with other bacterial cells, to alter the host’s response and environment and alternatively, it may form a biofilm (Distel et al, 2002).
Calcium hydroxide (Ca(OH)2) is currently the intracanal medication of choice. However its depth of penetration in the dentinal tubules is under scrutiny and several bacterial species, including E. faecalis, are reportedly resistant to its effects. Safavi et al (1990), and Siqueira and Uzeda (1996), suggested that Ca(OH)2 preparations placed in root canals for extended periods were unable to eliminate E. faecalis.
Chlorhexidine (CHX) has antimicrobial activity against Gram-positive and Gram-negative microorganisms. The antimicrobial effect of CHX is caused by the binding of the cationic molecule to negatively charged bacterial cell walls, thereby altering the cell's osmotic equilibrium. Its use in endodontics has been proposed both as an irrigant and as an intracanal dressing (Stuart et al, 2006; Zerella et al, 2005). Heling et al (1992), demonstrated that when it was used as an intracanal medicament, CHX was more effective than Ca(OH)2 in eliminating E. faecalis infection within dentinal tubules. Sukawat and Srisuwan (2002), demonstrated that Ca(OH)2 + 0.2% CHX has the same antimicrobial effect as Ca(OH)2 mixed with distilled water.
The purpose of this study was to evaluate over time, the antimicrobial effect of Ca(OH)2 + distilled water, Ca(OH)2 + CHX and CHX gel against E. faecalis present in the root canal of human teeth.
MATERIALS AND METHODS
Bacteriological Assay Seventy eight human maxillary anterior teeth were used throughout. The clinical crowns were removed using a diamond bur mounted on a high-speed handpiece with air-water coolants, at or near the cement-enamel junction, to obtain a standard root length of 15 mm. The working length was fixed to 14 mm by subtracting 1mm from the root length. The canals were instrumented with the step-back technique (Leonardo, 1994) up to a #50 master apical file (K-file Dentsply Maillefer, Ballaigues, Switzerland). After each instrument was used, the canals were irrigated with 1 ml of 1% NaOCl. The smear layer was removed by an ultrasonic bath in 17% EDTA for 5 min followed by an ultrasonic bath in 5.25% NaOCl for 5 min (Sukawat and Srisuwan, 2002). Each specimen was placed in 2 ml of Brain Heart Infusion (BHI) broth (Oxoid, Basingstoke, UK) and sterilized in autoclave for 15 min at 121°C. After cooling to room temperature, each specimen was incubated at 37°C for 48 h to confirm sterility.
A biofilm-forming activity bacterial strain15 E. faecalis ATCC 29212 was used in this study. It was sub-cultured for 24 h in BHI three times at 37°C. After the last incubation, 0.2 ml of E. faecalis suspension with the optical density adjusted to the turbidity of 1.5 x 108 colony-forming units (cfu) mL-1 were added to 2 ml of BHI containing each sterile dentin specimen and incubated at 37ºC for 24 h. This procedure was repeated for 3 d, renewing the broth every day. Broth contamination by bacteria other than E. faecalis was evaluated by examining the colony form and Gram stain after culturing on BHI agar.
After the infection period, the specimens were dried with sterile gauze, sterile paper point (Meta Dental Co., Seoul, Korea) and placed in sterile Petri dishes. The specimens were divided into 5 test groups of 15 specimens each, according to the intracanal medications used as follows:
Group 1: Ca(OH)2 (Anedra Lab., Buenos Aires, Arg) + distilled water (1:1, w/v)
Group 2: Ca(OH)2 + 2% CHX gluconate solution (ICN Biomedicals Inc, Ohio, USA) (1:1, w/v)
Group 3: 2% CHX gel (Endogel, Essencial Farma, Sao Paulo, Brazil)
Group 4: Positive control (infected, no medication)
Group 5: Negative control (uninfected, no medication)
The Ca(OH)2 pastes were placed using k-file #50. The file was rotated counterclockwise and withdrawn along the canal walls. This procedure was repeated until the canal was fully packed. The paste was further condensed with the aid of absorbent paper points and vertical pluggers (Estrela et al, 2002). The 2% CHX gel was placed employing the syringe and needle supplied. The coronal and apical parts of each specimen were sealed with Cavit.
Then, the external surfaces were disinfected for 10 min with 5.25% NaOCl. Concentration and action time were selected to guarantee adequate surface disinfection based on a previous study in which we assayed NaOCl concentrations between 1% and 5.25% and action times between 1min and 15 min. To evaluate disinfection, three extra infected specimens with no medication, not included in the test groups, were disinfected for 10 min with 5.25 % NaOCl and incubated in BHI 48 h at 37ºC.
The specimens were placed in Petri dishes on sterile cotton wool dampened with saline to create a humid environment. The dishes were placed in an incubation jar at 37°C for 1, 2, 4, 7 and 14 d. After each time three specimens of each test group were selected. The Cavit was eliminated and the medication was removed with file #50, irrigating with 20 ml of sterile distilled water (Safavi et al, 1990). The specimens were then immersed in 5 ml of BHI and incubated with agitation at 50 rpm and 37°C for 48 h. E. faecalis growth was evaluated by monitoring turbidity of the culture medium and confirmed by culture on Streptococcus Faecalis (SF) medium (Becton Dickinson and Company, Detroit, MI, USA) at 37°C for 48 h and by Gram stains. Specimen handling was performed under a laminar flow hood to avoid external contamination.
Scanning Electron Microscopic (SEM) Analysis Specimens for SEM analysis were processed simultaneously with the specimens for the in vitro bacteriological assay. After the infection period three specimens were processed to confirm presence of E. faecalis in the dentinal tubules. Forty five other specimens were dressed with the intracanal medicaments as in the 5 test groups and prepared for SEM analysis at 1, 7 and 14 d.
Longitudinal grooves were cut along the entire length of each root. The specimens were split with a hammer and chisel into two halves. Each half root was gently washed in 0.1 M phosphate buffer saline (PBS). The specimens were then fixed in 3.16% glutaraldehyde in 0.1 M phosphate buffer pH 7.4 for 24 h at 4°C, washed with PBS for 15 min, and post fixed in 1% osmium tetroxide for 12 h at 4°C. PBS was used as the final wash. Dehydration was performed in an ascending alcohol series, 50%, 70%, 80%, 90%, 100% (10 min washes), followed by 3 washes in acetone 100% (15 min washes). Specimens were dehydrated to their critical point with a drying apparatus (DCP-1, Denton, Tx, USA) using liquid CO2 replacement. Each specimen was mounted and coated with 200 Å layer of gold palladium. The coronal, middle and apical thirds of the root canals were observed in a JEOL JSM-35CF scanning electron microscope at 25 kV. Photographs were taken with an AGFA APX 100-120 film (Florencio Varela, Buenos Aires, Arg).
RESULTS
Bacteriological Assay The effects of intracanal medicaments are shown in Table 1. 2% CHX gel was effective in eliminating E. faecalis at 24 h, while Ca(OH)2 + distilled water and Ca(OH)2 + 2% CHX showed no antimicrobial effect on E. faecalis from 1 to 14 d.
No growth was seen in the negative control specimens. Bacterial growth was observed in the positive control until the end of the experiment.
The three specimens of each test group showed the same results at each time.
SEM Analysis SEM analysis showed E. faecalis in the dentinal tubules of human teeth after the infection period (Fig. 1). The negative control showed no bacterial colonization in the root canal until 14 d (Fig. 2).
Fig 1.
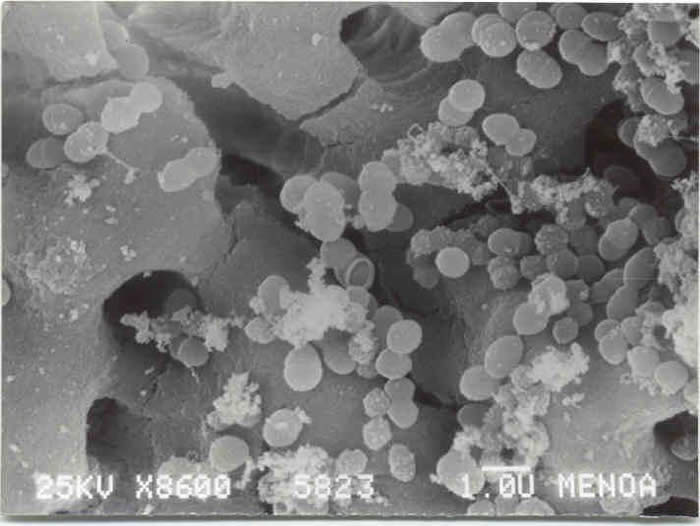
Fig 2.
Specimens treated with 2% CHX gel showed no bacteria at 1 d (Fig. 3a), at 7 d (Fig. 3b) and at 14 d (Fig. 3c).
Fig 3.
In teeth treated with Ca(OH)2 + distilled water bacteria were observed onto the root canal at 1 d (Fig. 4a), 7 d (Fig. 4b) and 14 d (Fig. 4c).
Fig. 4
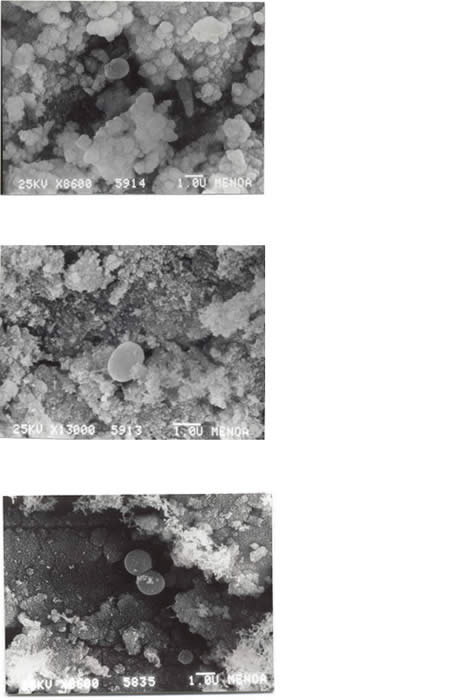
They were also observed in teeth treated with Ca(OH)2 + 2% CHX at 1 d (Fig. 5a), 7 d (Fig. 5b) and 14 d (Fig. 5c).
Fig. 5
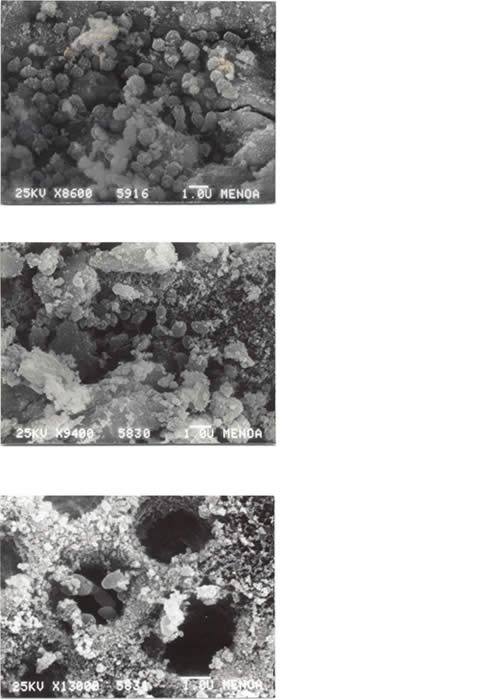
DISCUSSION
E. faecalis, a facultative anaerobic Gram-positive coccus, has been implicated in persistent root canal infection (Molander et al, 1998) and blamed for causing failure of root canal treatment. It is one of the most resistant microorganisms in root canal flora. In this study an ATCC strain previously used in vitro studies to evaluate the antibacterial effects of intracanal dressing (Distel et al, 2002; Estrela et al, 1999; Sukawat and Srisuwan, 2002) was selected.
Ca(OH)2, widely used for apexification and pulp capping procedure, is nowadays widely used as an intracanal medicament in endodontic therapy. Its high pH inhibits essential enzymes activities for metabolisms, growth, and cellular division of bacteria. The influence of pH alters the integrity of the cytoplasm membrane by disrupting organic components and transport of nutrients (Safavi et al, 1990). Ca(OH)2 was chosen for this study because it is currently the most popular intracanal medication. However, there is some controversy about its efficacy against E. faecalis. CHX is a cationic bisguanide that seems to act by adsorption to the cell wall of the microorganisms, causing leakage of intracellular components. At low CHX concentrations, small molecular weight substances will leak out, specifically potassium and phosphorous, resulting in a bacteriostatic effect. At higher concentrations, CHX has a bactericidal effect due to precipitation and/or coagulation of the cytoplasm, probably caused by protein cross-linking (Gomes et al, 2001; White et al, 1997). The tested CHX was used in the gel form (natrozole) to facilitate its application and prolong its retention in the root canal. Natrozole is a biocompatible carbon polymer, a water-soluble substance that can be completely removed from the root canal with a final flush of distilled water. It also has no antibacterial effect.
E. faecalis were observed in the root dentin of a positive control since the first day of infection until the end of the experiment, and was confirmed alive through its growth in a specific media for E. faecalis. Waltimo et al (2000), demonstrated that a very short incubation period was sufficient for the growth of E. faecalis in the dentinal tubules. Behnen et al (2001), demonstrated its ability to penetrate into the dentinal tubules of bovine teeth after 24 h incubation. Evans et al (2002), infected bovine root canals in 5 d. However, our study was not performed with the aim to demonstrate either adhesion or penetration of bacteria into tubules. It was just necessary to find at list one bacteria alive that can multiply in a specific growth media, in order to evidence the effectiveness of the antibacterial effect of an intracanal medicament. That is, the merely presence of bacteria makes unnecessary any quantitative analysis.
In this study 2% CHX gel was effective in the elimination of E. faecalis within dentinal tubules, while Ca(OH)2 + distilled water and Ca(OH)2 + CHX were ineffective in eliminating the bacteria. Gomes et al (2003), also demonstrated that 2% CHX gel alone was more effective against E. faecalis than Ca(OH)2. In our study the Ca(OH)2 and CHX combination showed the same antimicrobial effect as mixed with distilled water. These results are in agreement with those of Sukuwat and Srisuwan (2002), and Haenni et al (2003), CHX did not have a synergic effect on the antibacterial action of Ca(OH)2. Quite the opposite, the Ca(OH)2-CHX mixture, showed inhibition of the antibacterial activity of CHX. The possible reason for this reduced efficacy may be the deprotonation of the biguanide at pH > 10 and hence, a markedly reduced solubility and altered interaction with bacterial surfaces due to the change in the charge of the molecule. Controversially many authors demonstrated that Ca(OH)2 with CHX was more effective in eliminating E. faecalis from dentinal tubules than Ca(OH)2 with distilled water (Basrani et al, 2003; Delgado et al, 2010; Evans et al, 2003; Lin et al, 2003).
In human teeth, Siqueira and Uzeda (1993), and Ørstavik and Haapasalo (1990), reported that Ca(OH)2 was unable to completely eliminate E. faecalis from dentinal tubules after 10 d. Conversely, Han et al (2001), demonstrated that aqueous Ca(OH)2 paste was effective in the elimination of E. faecalis in the dentinal tubules after 7 d. Almiroundi et al (2002), demonstrated that Ca(OH)2 combined with distilled water killed E. faecalis within the dentinal tubules at 3 d and 8 d, but failed to do so at 14 d; the CHX gel and Ca(OH)2 combined with CHX was effective in eliminating E. faecalis inside dentinal tubules, as reported by the authors. These studies involved incubating dentin shavings or dentin powder after the action of the intracanal medicament. In our study, teeth were directly incubated following the methodology of Estrela et al (1999), who also demonstrated that Ca(OH)2 failed to eliminate E. faecalis from dentinal tubules after 7 d.
The antimicrobial action of Ca(OH)2 is related to the release of OH- ions in an aqueous environment and therefore depends on the availability of OH- ions in the solution and the ability of these ions to diffuse through dentin and pulpar tissue remnants to reach sequestered bacteria. Dentin itself has buffer properties for the base, so it might decrease the pH effect of the Ca(OH)2. Evans et al (2002), verified that the proton pump of the E. faecalis cell, which carries protons to the interior of the cell acidifying the cytoplasm, is important for the survival of the microorganism in an alkaline environment. Presumably, when the alkalinity of the environment reaches pH 11.5 or higher, this “lifeguard” mechanism is brought into action. This mechanism, associated to the dentin buffer properties, can explain the resistance of E. faecalis to Ca(OH)2 alone.
The use of an antimicrobial agent as an intracanal medication between appointments may increase the chances of a successful endodontic treatment by reducing residual bacteria in the root canal system.
Conclusions
The results of this study indicate that 2% CHX gel may exert an important role in the eradication of endodontic infection by E. faecalis associated with teeth that were refractory to the conventional endodontic therapy. Further clinical studies may help to confirm our in vitro findings.
REFERENCES
1. Almyroudi, A, Mackenzie, D, McHugh, S, Saunders, WP. (2002) The effectiveness of various disinfectants used as endodontic intracanal medications: an in vitro study. Journal of Endodontics. 28, 163-167.
2. Basrani, B, Tjäderhane, L, Santos, M, Pascon, E, Grad, H, Lawrence, HP, Friedman, S. (2003) Efficacy of chlorhexidine – and calcium hydroxide – containing medicaments against Enterococcus faecalis in vitro. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 96, 618-624.
3. Behnen, MJ, West, LA, Liewehr, FR, Buxton, TB, McPerson, JC. (2001) Antimicrobial activity of several calcium hydroxide preparations in root dentin. Journal of Endodontics. 27, 765-767.
4. Byström, A, Claesson, R, Sundqvist, G. (1985) The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol, and calcium hydroxide in the treatment of infected root canals. Endodontics and Dental Traumatology. 1, 170-175.
5. Delgado, RJ, Gasparoto, TH, Sipert, CR, Pinheiro, CR, Moraes, IG, García, RB, Bramante, CM, Campanelli, AP, Bernardinelli, N. (2010) Antimicrobial effects of calcium hydroxide and chlorhexidine on Enterococcus faecalis. Journal of Endodontics. 36, 1389-1393.
6. Distel, JW, Hatton, JF, Gillespie, MJ. (2002) Biofilm formation in medicated root canals. Journal of Endodontics. 28, 689-693.
7. Estrela, C, Pimenta, FC, Ito, YI, Bamman, LL. (1999) Antimicrobial evaluation of calcium hydroxide in infected dentinal tubules. Journal of Endodontics. 25, 416-418.
8. Estrela, C, Mamede Neto, I, Lopes, HP, Estrela, CR, Pécora, JD. (2002) Root canal filling with calcium hydroxide using different techniques. Brazilian Dental Journal. 13, 53-56.
9. Evans, MD, Davies, JK, Sundqvist, G, Figdor, D. (2002) Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. International Endodontic Journal. 35, 221-228.
10. Evans, MD, Baumgartner, JC, Khemaleelakul, SU, Xia, T. (2003) Efficacy of calcium hydroxide: chlorhexidine paste as an intracanal medication in bovine dentin. Journal of Endodontics. 29, 338-339.
11. Gomes, BPFA, Feraz, CCR, Vianna, ME, Berber, VB, Teixeira, FB, Souza-Filho, FJ. (2001) In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. International Endodontic Journal. 34, 424-428.
12. Gomes, BP, Souza, SF, Ferraz, CC, Teixeira, FB, Zaia, AA, Valdrighi, L, Souza-Filho, FJ. (2003) Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. International Endodontic Journal. 36, 267-275.
13. Haenni, S, Schmidlin, PR, Mueller, B, Sener, B, Zehnder, M. (2003) Chemical and antimicrobial properties of calcium hydroxide mixed with irrigating solutions. International Endodontic Journal. 36, 100-105.
14. Han, GY, Park, SH, Yoon, TC. (2001) Antimicrobial activity of Ca(OH)2 containing pastes with Enterococcus faecalis in vitro. Journal of Endodontics. 27, 328-332.
15. Heling, I, Steinberg, D, Kening, S, Gavrilovich, I, Sela, MN, Friedman, M. (1992) Efficacy of a sustained-release device containing chlorhexidine and Ca(OH)2 in preventing secondary infection of dentinal tubules. International Endodontic Journal. 25, 20-24.
16. Leonardo, MR. (1994) Preparación biomecánica de los conductos radiculares. In: Leonardo, MR, Leal, JM, editors. Endodoncia: Tratamiento de los conductos radiculares. Argentina: Buenos Aires. 296-320.
17. Lin, Y, Mickel, AK, Chogle, S. (2003) Effectiveness of selected materials against Enterococcus faecalis: Part 3. The antimicrobial effect of calcium hydroxide and chlorhexidine on Enterococcus faecalis. Journal of Endodontics. 29, 565-566.
18. Love, RM. (2001) Enterococcus faecalis – a mechanism for its role in endodontic failure. International Endodontic Journal. 34, 399-405.
19. Molander, A, Reit, C, Dalhén, G, Kvist, T. (1998) Microbiological status of root-filled teeth with periodontitis. International Endodontic Journal. 31, 1-7.
20. Ørstavik, D, Haapasalo, M. (1990) Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endodontics and Dental Traumatology. 27, 218-222.
21. Peciuliene, V, Balciuniene, I, Eriksen, H, Haapasalo, M. (2000) Isolation of Enterococcus faecalis in previously root-filled canals in Lithuanian population. Journal of Endodontics. 26, 593-595.
22. Safavi, KE, Spångberg, LS, Langeland, K. (1990) Root canal dentinal tubule disinfection. Journal of Endodontics. 16, 207-210.
23. Siquiera, JF Jr, Uzeda, M. (1996) Disinfection by calcium hydroxide pastes of dentinal tubules infected with two obligate and one facultative anaerobic bacteria. Journal of Endodontics. 22, 674-676.
24. Sjögren, U, Fidgor, D, Spångberg, L, Sundqvist, G. (1991) The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. International Endodontic Journal. 24, 119-125.
25. Stuart, CH, Schwartz, SA, Beeson, TJ, Owatz, CB. (2006) Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retirement. Journal of Endodontics. 32, 93-98.
26. Sukawat, C, Srisuwan, T. (2002) A comparison of the antimicrobial efficacy of three calcium hydroxide formulations on human dentin infected with Enterococcus faecalis. Journal of Endodontics. 28, 102-104.
27. Waltimo, TMT, Ørtavik, D, Siren, EK, Haapasalo, MPP. (2000) In vitro yeast infection of human dentin. Journal of Endodontics.26, 207-209.
28. White, RR, Hays, GL, Janer, LR. (1997) Residual antimicrobial activity after canal irrigation with chlorhexidine. Journal of Endodontics. 23, 229-231.
29. Zerella, JA, Fouad, AF, Spangberg, LS. (2005) Effectiveness of calcium hydroxide and chlorhexidine digluconate mixture as disinfectant during retreatment of failed endodontic cases. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 100, 756-761.
FIGURES LEGENDS
Fig 1: SEM of E. faecalis in dentinal tubules of human teeth after the infection period Positive control)
Fig 2: SEM of dentinal tubules of uninfected human teeth with no medication (Negative control)
Fig 3: SEM of root canal medicated with 2% CHX gel a) 24 h b) 7 d c) 14 d
Fig 4: SEM of E. faecalis in root canal medicated with Ca(OH)2 + distilled water a) 24 h b) 7 d c) 14 d
Fig 5: SEM of E. faecalis in root canal medicated with Ca(OH)2 + 2% CHX a) 24 h b) 7 d c) 14 d
ACKNOWLEDGEMENTS
This study was partially supported by a grant from CIUNT (Consejo Nacional de Investigaciones Científicas y Técnicas de la Universidad Nacional de Tucumán). The authors would like to thank Ing Alberto Andrada Barone of the Laboratory of Electronic Microscopy of INSIBIO (Instituto de Investigaciones Bioquímicas) for suggestions on SEM.
DISCLOSURE OF COMMERCIAL INTERESTS
Authors have no financial involvement that might present an appearance of conflict of interests.
Copyright Priory Lodge Education Limited 2013-
First Published January 2013
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


