Browse through our Journals...
TUBERCULOSIS IN CHILDHOOD WHEEZING-A RETROSPECTIVE ANALYSIS
Dr. Sukhbir K. Shahid, MD
Consultant Pediatrician and Neonatologist, Mumbai-77
SUMMARY
Background and Objectives: Tuberculosis can cause bronchospasm but it is often misdiagnosed as asthma. We reviewed medical records of wheezing children attending our clinic to estimate percentage, demographic and clinical features of wheezing children with coexistent TB.
Methods: Wheezing children were grouped into those with associated active TB, and those with no TB. Demographic details, clinical presentation, age of onset of wheeze, wheeze severity and control were evaluated in these two subgroups and compared for statistical significance, if any.
Results: 300 wheezing children attended our clinic over the past 5 years. 52 (17.3%) of wheezing children had associated active tuberculosis. In these children, mean age of onset of wheeze was significantly later compared to TB-negative wheezers (3.6±0.3 vs. 2.0±0.1 years, p<0.05). This delay was more marked in non-atopic TB wheezers with no genetic predisposition to wheeze. 85.7% of children with TB and asthma who were followed up for at least 2 years, were able to stop their anti-asthma medicines. Whereas in non-TB wheezers, who had follow-up of up to 2 years, only 63.5% could cease their anti-asthma treatment (p<0.05)
Conclusion: Active tuberculosis in childhood wheezing is not uncommon. Children with this dual illness present significantly later compared to wheezers with no TB. There are no specific clinical pointers towards likelihood of TB-induced wheeze in children and only a high index of suspicion with relevant tests and its treatment, would aid in early and better control of wheeze in these children.
INTRODUCTION
Wheezing illnesses are one of the major causes of emergency visits and hospitalizations in children. Globally around 3-30% of children suffer from this airway disorder (Busquets RM et al, 1996, Abuekteish F et al, 1996, Martinez FD, 1995). This encompasses a heterogeneous group which includes asthmatics, as well as those with underlying problems such as a foreign body, viral infections, aspiration pneumonia, tuberculosis, congenital heart disease, bronchiectasis, sinusitis or cystic fibrosis. These secondary causes of wheeze need to be detected and tackled simultaneously for better outcomes in these children (Go RO et al, 1997).
Tuberculosis is widely prevalent in tropical countries. In India, its prevalence is on the rise due to a number of reasons (Prabhakar R, 1996, Starke JR et al, 1992). It is a not so uncommon cause of wheeze in children leading to a misdiagnosis of asthma in them (Novello A et al, 1994, Lin S et al, 1996). Wheeze in them is possibly due to compression of trachea and major airways by enlarged hilar lymph nodes (Niggemann B et al, 1995). In 1/3-1/4 percent of cases of endobronchial tuberculosis in children wheezing episodes may be present, and in this form of TB pulmonary function tests are normal (Park CS et al, 1995, Daly JF et al, 1952). Allergenic antigens of the mycobacterium may also be responsible for the bronchoconstriction by a mechanism similar to that in allergic conjunctivitis (phylectenular conjunctivitis), synovitis and TB encephalopathy (Banapurmath CR et al, 1998, Bellendir EN, 1994, Char G & Morgan OS 2000) . The fortunate thing is that tuberculosis is completely curable and hence it is likely that TB-induced wheeze may also be cured with optimal anti-kochs treatment.
We carried out a retrospective analysis of medical data of wheezing children attending our clinic to evaluate the percentage of children with co-existent TB and to know whether wheezing children with co-existent tuberculosis differed demographically or clinically from other wheezers with no active tuberculosis.
SUBJECTS AND METHODS
We analyzed medical records of recurrently wheezing children who had attended our clinic in the last 5 years. Recurrent wheeze was considered to be present when child had 3 or more episodes of wheeze. Detailed history and clinical examination findings were noted down. History of precipitating factors was looked into and these were grouped into following 7 categories: aeroallergens (like dust, tree pollens, perfumes, smoke), viral cold, food allergens (such as fruits, curds, fried foods), cold bath (including hair bath), weather-related (such as weather change, rains, cold weather), exercise and emotions. Birth history, family history of asthma and smoking habits of family members was dealt into. Anthropometric details of each child were jotted. Severity of wheeze in these children was estimated by International Pediatric Asthma Consensus Group Report 1992 (Anon. 1992). Accordingly, patients were categorized as mild, moderate or severe wheezers. Initial routine screening for TB in form of complete blood count, X-ray chest and Mantoux test (MT) is performed on all wheezing children attending our clinic. Mantoux test was carried out with 0.1 ml of 1 TU PPD with Tween 80 and reading was taken 72 hours after administration of test. Diagnosis of active tuberculosis was based on positive MT and chest X-ray and suggestive symptomatology and clinical examination. Interpretation of Mantoux test and chest skiagram was done using the standardized methods (Udani and Somu, 1996, Woodring et al, 1986). Positive x-ray findings included hilar lympadenopathy, unresolving pulmonary infiltrates, cavities, and pleural collections (Leung et al, 1992). Other relevant tests such as gastric aspirate for acid-fast bacilli, high resolution CTscan chest were done where indicated ( Starke JR, 2004). Patients with associated tuberculosis were initiated on anti-koch’s therapy, along with bronchodilators and steroids (based on the severity of their asthma).
We grouped our wheezing children into: those with co-existent tuberculosis and those who were TB-negative by our baseline investigations. Demography, clinical symptoms, mean age of presentation and severity of wheezing in children in these two subgroups were evaluated and compared for statistical difference, if any. The percentage of children who could stop anti-wheeze therapy by 2 years of registration and management were evaluated in these children and compared.
Statistical Analysis
All data are expressed as mean ± SEM. Statistical analysis was done by the Chi-square test and the statistical analysis of difference between two means. A p value of < 0.05 was considered as statistical significance (Munro BH, 2001).
RESULTS
We had 300 children who had attended our outpatients for repetitive wheezing episodes in last 5 years (171 females, mean age 4.8 ±0.2 years). Mean age of onset of wheeze was 2.1± 0.1 y (range=6 m to 11.5 y). The patients in mild, moderate and severe grades were 87 (29%), 167 (55.7%) and 46 (15.3%) respectively.
Past history of TB was elicited in 20 (6.7%) children with wheezing. On initial screening, 52 wheezing children had evidence of co-existent present tuberculosis. Demographic features of wheezing children with and without TB are as shown in Table 1. Likely precipitating factors in these two groups of children are as depicted in Table 2. 143 children with TB-negative and 32 with TB-associated wheeze had history of more than 1 likely precipitating factors for wheeze.
52 (100%) children in TB group had respiratory distress and 30 (57.7%) had associated cough. Only two children in this group complained of fever. In the non-TB group, 246 (100%) children had complaints of respiratory distress and 132 (53.2%) had associated cough. Two of these children had fever as a significant symptom. 90.4% of children with TB-associated wheeze had positive MT, and 63.5% of children of them had hilar lympadenopathy on x-ray chest. 2 children had endobronchial tuberculosis. Gastric aspirate/sputum analysis was done in 10 children and it was found to yield acid-fast bacilli in 3 children with TB-associated wheeze. HRCT chest was required to be done in 3 cases and showed significant bronchiectasis in 1 child. Repeat HRCT chest done in this child after 1 year of optimal treatment showed more than 50% reversal of bronchiectasis.
??
Mean age of onset of wheeze in TB subgroup was significantly later than that in non-TB subgroup of wheezers (3.6±0.3 vs. 2.0±0.1 years respectively, p<0.05). This difference remained significant when non-atopic TB wheezes were compared with atopic non-TB-associated wheezes. ( 3.6 ± 1.0 vs 2.0 ± 0.1 respectively, p<0.05) (Table 3).
Severity of wheeze in non-TB and TB wheezers was comparable. Atopy also did not seem to affect the severity of wheeze in children with no TB. (Table 3).
Family history of contact with TB was present in 28.3% of cases; 76.9 and 18.1% in TB wheezers and non-TB associated wheeze respectively. Family history of wheezing was present in 26.9 and 28.6% of children with TB and TB-negative wheezers respectively (p>0.05). Children with family history of wheeze and with no TB had earlier onset of wheeze compared to those with TB and no family history of allergy on history (1.6 ± 0.1 vs 2.9 ± 0.3 y respectively, p=0.0002). Wheeze severity remained unaffected by positive family history in children with no TB. (Table 4). Non-TB atopic children with positive family history of wheezing had earlier onset of wheeze compared to TB children who were non-atopic and had no genetic predisposition (1.8 ± 0.1 vs 3.6 ± 1 y respectively, p=0.01). The severity of the illness did not vary between these 2 subsets of patients.
Passive exposure to bidi and/or cigarette smoking was seen in 50 and 43.5% of wheezing children with TB and non-TB wheezers respectively (p>0.05). This exposure did not affect the age of onset of severity of wheeze in children in TB and non-TB group.
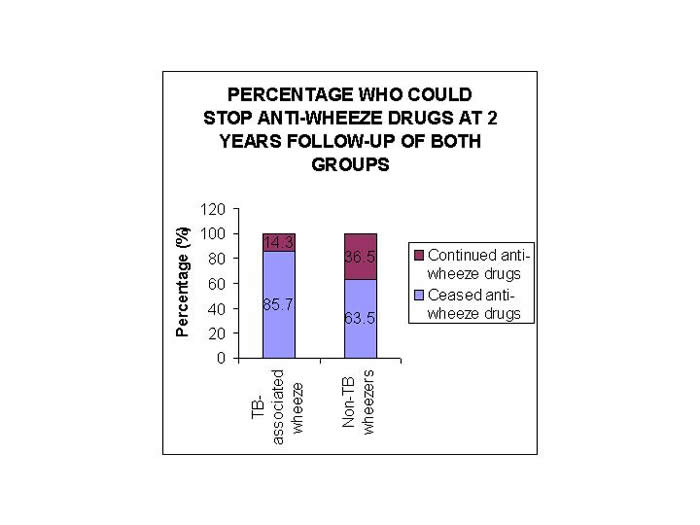
49 of these children could be followed up for at least 2 years, and out of these, 85.7% of children could stop their anti-asthma medicines by 2 years. Duration of anti-wheeze treatment in them ranged from 6 m to 1.7 y. Whereas in non-TB wheezers, 230 children had follow-up up to at least 2 years and only 63.5% of children could cease their anti-asthma treatment (p<0.05) (Figure 1).
DISCUSSION
Our retrospective analysis has shown that wheezing children with co-existent tuberculosis and no history of allergy had a later onset of wheeze compared to non-TB wheezers. There were no other definite clinical pointers to suspect TB in these children and only a high index of suspicion would help to pick up causative/associative tuberculosis in them. The impact of TB on age of onset and severity of wheeze has been analyzed for the first time in this study. Also follow-up of wheezing children over 2 years was done in this study and it showed that wheezing children with TB had better control of their bronchospasm and a significant number could cease their anti-wheeze medications.
This study highlights effect of associated tuberculosis on wheezing in children. It can be seen that wheezes in TB-diseased children present later compared to non-TB wheezes. There is better outcome in course of reactive airway disease in wheezing children with TB, but severity of their wheeze pattern does not vary significantly compared to non-TB wheezes. Our study also revealed that atopy and genetic predisposition to wheeze does seem to prepond the onset of wheeze, though these two do not seem to have any bearing on the severity of wheezing illness in these children. This, being a retrospective analysis, cannot control certain extraneous unknown factors which might have confounded the results. Besides, diagnosis of tuberculosis in wheezing children does not imply that it is the causative factor for the wheeze. It may be just an association or an aggravating factor. Our study cannot differentiate between these two subsets of wheezing children with tuberculosis. We did not carry out extensive diagnostic tests for tuberculosis in these children. Further research in this direction using tuberculosis antibody assays may be beneficial to understand the complex relationship between TB and wheezing, and to develop an algorithm of set of clinical and laboratory tests for identification of TB-induced wheezing.
Pulmonary tuberculosis in children can present in the typical way, but in a sizeable proportion of diseased individuals, it may mimic other diseases. Pereira K.D. et al has reported a case in which TB has masqueraded as a foreign body (Pereira KD et al, 1997). The primary complex of tuberculosis consists of the Ghon’s focus; pulmonary reaction with the draining lymphatics and the hilar and paratracheal lymph nodes. The enlarged lymph glands can compress the major bronchi and elicit a wheeze. El Nawaway et al found out that many of their wheezy children was in fact wheezing due to TB (el-Nawawy AA et al, 2000). In a country where tuberculosis has reached an alarming proportion, the prevalence of wheeze due to tuberculosis is also expected to be more. In the western world, atopy and allergies to the various environmental factors cause wheezing illnesses. Viral infections are also responsible for wheeze in younger children (Johnston SL, 1999). Tuberculosis is often missed and mistaken for asthma because it is not considered (Davies PDO, 2002). In India, there are no definitive statistics about the proportion of the various causes of wheeze. Viral infections do cause wheeze in toddlers, but bacterial infections may also be responsible for an equal, if not more number of wheezes in children (Seear M & Wensley D, 1997). In our study, TB was found to be associated in 17.3% of the cases, which means that 1 in every 5 wheezing children may have associated TB, though one cannot be sure in this analysis as to whether TB itself was responsible for the wheeze in children. Fever was seen in a small percentage of these children. Similar findings have been observed by other studies (Daly JF, 1952, Marais B J et al, 2005). Pulmonary TB accounts for 78.9% of cases of childhood TB (nelson LJ et al, 2004). Schaaf et al also found that children with confirmed respiratory TB had symptoms and signs not much different from those with other non-TB respiratory illnesses (Schaaf HS, 1995).
When we did the follow-up of these patients, significantly more percentage of the patients who had wheeze and TB could stop their anti-asthma medications than their counterpart wheezers who had no associated TB. But we also know that pick-up of the TB diseased cases is not 100% and some in the only wheeze group may have had undiagnosed or latent TB (Khan EA & Starke JR, 1995, Schluger NW, 2001). Also some in the TB and wheeze group might have had a co-incident TB infection or disease which is not responsible for the wheeze. A follow-up of these cases over the years would help to clear this. But we do not have such a long follow-up in our study.
The complex interrelationship between tuberculosis and wheezing is emphasized by the fact that though tuberculosis can cause wheeze, it can also lead to decrease in wheeze in patients with past history of treated tuberculosis. TB activates Th1 response and this in turn down-regulates Th2 immune response which is responsible for allergy and asthma (von Mutius E et al, 2000, von Hertzen L et al, 1999). This is supposedly the reason for the high prevalence of asthma in countries with low incidence of TB. The level of immunoglobulin E in the blood has also been shown to decrease with anti-Koch’s treatment, implying that as immunity towards the infection mounts, the atopic tendency declines (Ohrui T et al, 2000). This belief has reached an extent wherein possibility of TB vaccine as preventive measure for asthma is being echoed (Scanga CB & Le Gros G, 2000). But wheeze may definitely be elicited in patients with TB during the time that they have active disease. Hence in countries with high prevalence of TB, investigations in wheezing children should definitely include tests to rule out tuberculosis. This testing for TB in wheeze is especially important as steroids now for the mainstay of therapy in asthma. If associated TB remains undetected, it theoretically would flare up and cause disseminated forms of TB. This would not be pardonable. Also given to understand the stigma, panic and chronicity tagged with the diagnosis of asthma, ruling out transient causes of wheeze such as TB is vital.
Tuberculosis should continue to be an essential part of the initial battery of investigations in children with wheezing. Thorough and proper workup should not be ignored in pediatric wheezing. The dictum of considering pediatric wheezing as being due to allergy/viral infections should be avoided. Considering the better control and cure of wheezing disorder in children with tuberculosis and asthma, diagnosis and management of co-existent tuberculosis in these patients would be useful.
Further prospective studies are indicated to study the influence of TB on wheeze in children and to establish an algorithm for easy identification of children with TB-induced wheeze, especially in view of the fact that TB-induced wheezes have higher likelihood of control and cure. Large-scale studies coupled with newer specific diagnostic tests for TB would be beneficial.
CONCLUSIONS
This retrospective evaluation has revealed that TB associated wheeze is responsible for a significant proportion of children with wheezing. This wheeze has a delayed onset of symptoms. Also, it is better controlled and cessation of anti-wheeze treatment is more likely in this group of cases. Severity of wheeze however has no correlation with cause of wheeze.
Competing Interests:
The author declares that she has no competing interests
BIBLIOGRAPHY:
- Abuekteish, F, Alwash, R, Hassan, M, Daoud, AS. (1996) Prevalence of asthma and wheeze in primary school children in Northern Jordan. Ann Trop Paediatr 16(3), 227-231.
- Anon. (1992) International Consensus Report on diagnosis and treatment of asthma. National Heart, Lung, and Blood Institute, National Institutes of Health. Bethesda, Maryland 20892. Publication no. 92-3091. Eur Respir J 5(5), 601-604.
- Banapurmath, CR, Varghese, A, Koujalgi, MB. (1998) Phlyctenular conjunctivitis. Indian Pediatr 35(6), 561
- Bellendir, EN, Shenderova, RI, Goriashina, VI, Ikunova, OA, Nakonechny, GD. (1994) Pathogenetic rationale and diagnostic methods in allergic tuberculous synovitis of the knee joint. Probl Tuberk 3, 34-38
- Busquets, RM, Anto, JM, Sunyer, J, Sancho, N, Vall, O. (1996) Prevalence of asthma-related symptoms and bronchial responsiveness to exercise in children aged 13-14 yrs in Barcelona, Spain. Eur Respir J 9(10), 2094-2098.
- Char, G, Morgan, OS. (2000) Tuberculous encephalopathy. A rare complication of pulmonary tuberculosis. West Indian Med J 49(1), 70-72
- Daly, JF, Brown, DS, Lincoln, EM, Wilking, VN. (1952) Endobronchial tuberculosis in children. Chest 22, 380-398.
- Davies PDO. The challenge of tuberculosis. Infection 2002. http://www.priory.com/cmol/tb2000.htm
- el-Nawawy, AA, Massoud, MN, el-Nazzar, SY, Ramy, BB. (2000) Pulmonary tuberculosis as a cause of recurrent wheezy chest: the value of serological diagnosis using IgG antibodies to mycobacterium 38 kDa antigen [letter]. J Trop Pediatr 46(1), 53-54.
- Go, RO, Martin, TR, Lester, MR. (1997) A wheezy infant unresponsive to bronchodilators. Ann Allergy Asthma Immunol 78(5), 449-456
- Johnston, SL. (1999) The role of viral and atypical bacterial pathogens in asthma pathogenesis. Pediatr Pulmonol Suppl 18, 141-143.
- Khan, EA, Starke, JR. (1995) Diagnosis of Tuberculosis in Children: Increased Need for Better Methods. Emerging infectious diseases 1 (4), 115-123.
- Leung, AN, Muller, N, Pineda, PR, FitzGerald, GM. (1992) Primary tuberculosis in children: radiographic manifestations. Radiology 182, 87-91.
- Lin, S, Peng J, Wang Z. (1996) A sample survey and multiple factor analysis on asthma in urban area of Shanghai (abstract). Chung Hua Chieh Ho Ho Hu Hsi Tsa Chih. 19 (1), 11
- Marais, B J et al. (2005) The prevalence of symptoms associated with pulmonary tuberculosis in randomly selected children from a high burden community. Arch Dis Child 90, 1166-1170
- Martinez, FD, Wright, AL, Taussig, LM, Holberg, CJ, Halonen, M, Morgan, WJ. (1995) Asthma and wheezing in the first six years of life. N Engl J Med. 332, 133-138.
- Munro BH. (2001) Statistical methods for health care research. 4th ed. Philadelphia, Lippincott.
- Nelson, LJ, Schneider, E, Wells, CD, Moore, M. (2004) Epidemiology of Childhood Tuberculosis in the United States, 1993–2001: The Need for Continued Vigilance. Pediatrics. 114, 333-341
- Niggemann, B, Klettke, U, Magdorf, K, Wahn, U. (1995) Two cases of pulmonary tuberculosis presenting with unilateral pulmonary hyperinflation in infancy. Eur J Pediatr 154(5), 413-415.
- Novello, A, Talenti, E, Barbato, A. (1994) Misdiagnosis of asthma. Two paradigmatic case reports. Minerva Pediatr 46(3), 113-116.
- Ohrui, T, Zayasu, K, Sato, E, Matsui, T, Sekizawa, K, Sasaki, H. (2000) Pulmonary tuberculosis and serum IgE. Clin Exp Immunol 122(1), 13-15
- Park, CS, Kim, KU, Lee, SM, Jeong, SW, Uh, SS, Kim, HT, Kim, YH. (1995) Bronchial hyperreactivity in patients with endobronchial tuberculosis. Respir Med 89(6), 419-422
- Pereira, KD, Mitchell, RB, Eyen, TP, Lazar, RH. (1997) Tuberculous lymphadenopathy masquerading as a bronchial foreign body. Pediatr Emerg Care 13(5), 329-330.
- Prabhakar, R. (1996) Tuberculosis--the continuing scourge of India. Indian J Med Res 103, 19-25.
- Scanga, CB, Le Gros, G. (2000) Development of an asthma vaccine: research into BCG. Drugs 59 (6), 1217-1221
- Schaaf, HS, Beyers, NN, Gie, RP, Nel, ED, Smuts, NA, Schoslash, FE, Donald, PR, Fourie, PB. (1995) Respiratory tuberculosis in childhood: the diagnostic value of clinical features and special investigations. Ped Infect Dis J. 14 (3), 189-194
- Schluger NW. (2001) Changing approaches to the diagnosis of tuberculosis. Am. J. Respir. Crit. Care Med. 164, 2020-2024
- Seear, M, Wensley, D. (1997) Chronic cough and wheeze in children: do they all have asthma? Eur Respir J 10(2), 342-345.
- Starke, JR, Jacobs, R, Jereb, J. (1992) Resurgence of tuberculosis in children. J Pediatr 120, 839-855.
- Starke, JR. (2004) Tuberculosis in Children. Semin Respir Crit Care Med 25, 353-364
- Udani, PM, Somu, N. (1996) Tuberculosis in children. Clinical features and presentation. In childhood tuberculosis, Lupin publication, 18-20.
- von Hertzen, L, Klaukka, T, Mattila, H, Haahtela, T. (1999) Mycobacterium tuberculosis infection and the subsequent development of asthma and allergic conditions. J Allergy Clin Immunol 104(6), 1211-1214.
- von Mutius, E, Pearce, N, Beasley, R, Cheng, S, von Ehrenstein, O, Bjorksten, B, Weiland, S. (2000) International patterns of tuberculosis and the prevalence of symptoms of asthma, rhinitis, and eczema. Thorax 55(6), 449-453.
- Woodring, JH, Vandiviere, HM, Fried AM, et al. (1986) Update: The Radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol. 146, 497-506.
Figure 1: Proportion of wheezing children on anti-asthma drugs at 2 years of follow-up
Table 1: Demographic details of wheezing children
________________________________________________________________________________
Parameters TB and wheeze group Non-TB wheezing group
________________________________________________________________________________
Number 52 248
Mean age (years) 5.7±0.4 4.7±0.2*
Females 28 143
Preterm delivery 2 (3.8) 11 (4.4)
Family history of asthma 14 (26.9) 71(28.6)
Passive smoking 26 (50) 108 (43.5)
Mean age of onset
of wheeze 3.6±0.3 2.0±0.1*
Mild wheeze 14 (26.9) 73 (29.4)
Moderate wheeze 30 (57.7) 137 (55.2)
Severe wheeze 8 (15.4) 38 (15.3)
Nutritional status(% normal) 81.4±1.7 79.7±0.7
________________________________________________________________________
* p<0.05
Numbers in parenthesis represent %.
Table 2: History of precipitating factors in wheezing children
________________________________________________________________________________
Parameter TB wheezers Non-TB wheezers
(n=52) (n=248)
________________________________________________________________________________
Viral cold 6 (11.5) 32 (12.9)
Aeroallergens 5 (9.6) 30 (12.1)
Food allergens 34 (65.4) 156 (62.9)
Weather-related 25 (48.1) 109 (44)
Cold bath 21 (40.4) 102 (41.1)
Exercise 6 (11.5) 31 (12.5)
Emotions 1 (1.9) 13 (5.2)
________________________________________________________________________
Figures in parenthesis are %
Table 3: Impact of atopy on age of onset and severity of wheeze:
________________________________________________________________________________
Parameter TB and non-atopic Non-TB and atopic p value
n=6 n=216
________________________________________________________________________________
Mean age of onset (y) 3.6 ± 1.0 2.0 ± 0.1 <0.05
Mild (%) 3 (50) 61 (28.2) >0.05
Moderate (%) 3 (50) 122 (56.5) >0.05
Severe (%) 0 (0) 33 (15.3) >0.05
________________________________________________________________________________
Table 4: Family history of wheeze and TB:
Parameter TB and negative family Non-TB and positive family p value
history (n=38) history (n=71)
Mean age of onset
(y) 2.9 ± 0.3 1.6 ± 0.1 0.0002
Mild (%) 12 (31.6) 17 (23.9) >0.05
Moderate (%) 21(55.3) 45 (63.4) >0.05
Severe (%) 5 (13.1) 9 (12.7) >0.05
________________________________________________________________________________
Copyright Priory Lodge Education Ltd 2008
First Published March 2008
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


