Browse through our Medical Journals...
Th2-shift in schizophrenia: primary findings from whole blood in vitro stimulation
Sonnig Sue Whei Chiang, Michael Riedel, Norbert Müller, Manfred Ackenheil, Rudolf Gruber, Markus Schwarz
Psychiatric Hospital of University Munich, Germany
Nussbaumstr. 7, 80336 Munich, Germany
Abstract
Motive: Newly, Th2-shift was suggested as a possible pathogenic mechanism in schizophrenia. Direct empirical evidence in this regard is, however, lacking. Aim: This study attempted to examine whether Th2-shift occurred in schizophrenic patients. Th2-shift was defined as significantly reduced IFN-g/IL-4 and/or IFN-g/IL-10 ratio, compared to healthy controls. Methods: Subjects: 44 schizophrenics and 76 controls were recruited. Cytokine measurement: Cytometric Bead Array (CBA) with a possibility to measure more cytokines simultaneously was used to assess in vitro cytokine production. Statistics: The data was analyzed by using Multi-variance-(co-variance) analysis (MAN(C)OVA). Results: (1) Schizophrenics as a whole group demonstrated a noticeably reduced IFN-g/IL-10 ratio. (2) Female schizophrenics had markedly decreased IFN-g/IL-10; in addition, the reduction in IFN-g/IL-4 ratio also showed a trend to be significant. Nevertheless, (3) no clear change regarding IFN-g/IL-4 and IFN-g/IL-10 ratio was found in male patients. Conclusion: Whole blood assay Th1/Th2 imbalance in favor of Th2-shift in schizophrenia seemed to be more likely observed in female schizophrenic patients.
Key words: Th2-shift, schizophrenia, cytokine, hormones, whole-blood-assay.
Introduction
Several lines of evidence suggest alterations of the immune system in schizophrenic patients. The fact that treatment with an immunomodulatory drug shows beneficial effects on the symptomatology of schizophrenia indicates immune dysfunction in schizophrenia is not just an epiphenomenon, but related to the pathomechanism of this disorder (Muller et al., 2004). In determining an immune response, the balance between two T helper lymphocyte subpopulations, Th1 and Th2, is believed to be pivotal and is therefore critical for the host defense and the pathogenesis of immune-mediated diseases (Agnello et al., 2003; McGuirk and Mills, 2002). The key Th1 cytokine, IFN-g, which is requisite for the development of the Th1 system, was found to be significantly reduced in distinct schizophrenic subgroup (Rothermundt et al., 2000; Arolt et al., 2000). Similar findings relating to another Th1 cytokine, IL-2, were also described (Zhang et al., 2002; Arolt et al., 2000). Hypo-production of the Th1 cytokines stated above could be caused by over-activation of the Th2-system. Thus, Th2-shift was recently suggested as a possible pathogenesis in at least a subgroup of schizophrenics (Schwarz et al., 2001a; Schwarz et al., 2001b; Muller et al., 2000). Publications concerning Th2 cytokine in vitro production in schizophrenics are to date rare. The only one study investigated the characteristic Th2 cytokine, IL-4, which is essential for the Th2 differentiation and development, showing an insignificant enhancement (Wilke et al., 1996). A few studies examined another main Th2 cytokine, IL-10, and demonstrated to be either unaltered (Cazzullo et al., 2002; Rothermundt et al., 1998) or augmented (Cazzullo et al., 1998).
However, reduced IFN-g or enhanced IL-4 or increased IL-10 alone doesn’t inevitably mean Th2-shift if the major Th1/Th2 cytokines of the same subject were evaluated separately. So far, no investigation scrutinizes Th1/Th2 balance in schizophrenia, simultaneously considering the indicators of both Th-systems in the same individual. Therefore, this study aims at examining whether a Th2-shift happens in schizophrenics, calculating the ratio between IFN-g and IL-4 as well as that between IFN-g and IL-10 in each study participant.
In addition, although the reciprocal correlations between the endocrine and immune system are nowadays well-known (Egger, 1992), reports pertaining to cytokine secretion in schizophrenics and simultaneously involving distinct endocrinological parameters are absent. In the present study, Th1/Th2 imbalance in schizophrenics was hence examined under the co-variation of stress hormones such as cortisol and prolactin as well as sex-hormones including estradiol, testosterone, and the sex-hormone-binding globuline (SHBG). The attempt to include those endocrinological variables as co-variants was to control whether Th1/Th2 imbalance in schizophrenia is rather a result of disease process or just the effects of various hormones since those hormones were present in whole blood as it was stimulated to produce cytokines in vitro. Furthermore, most important of all, those endocrinological factors mentioned above were found to have influences on Th1/Th2 cytokines such as IFN-g, IL-4, IL-10, IL-12, and IL-2 (Dimitrov et al., 2004; Xie et al., 2002; Elenkov and Chrousos, 2002; Miyaura and Iwata, 2002; Angele et al., 2001; Huber et al., 1999). SHBG, a protein binding to testosterone and estradiol with high affinity, might also have impacts on Th1/Th balance directly or indirectly via its relationships towards both sex-hormones. So, cortisol, prolactin, testosterone, estradiol, and SHBG were measured and included into the analysis as co-variants if they were able to clearly distinguish schizophrenics from healthy subjects in order to control their effects on Th1/Th2 ratios.
Methods
Subjects
This study included 44 schizophrenic patients (18 females and 26 males) as well as 76 healthy subjects (35 women and 41 men) who were between 18 and 61 years old (range: schizophrenia 18-61; controls 18-60 yrs old). The mean ages of the whole schizophrenic group, female, and male schizophrenic patients were 36.25 (SD = 12.68), 39.17 (SD = 12.39), and 34.23 (SD = 12.72) years old, while those for the whole healthy subjects, female, and male controls were 29.75 (SD = 8.39), 29.34 (SD = 7.84), and 30.10 (SD = 8.91) years old, respectively. All participants had given their written informed consents to take part into the study. The reason why there were double so many control subjects as schizophrenic patients was that healthy controls represent rather a general population that contains various relatively homogenous subpopulations such as schizophrenics. A general population usually has a greater variance than a subpopulation concerning the magnitudes of different biological features. Because this is the first study comparing the Th1/Th2 ratios between schizophrenia and normal controls, almost twice as many healthy controls as schizophrenic patients were recruited in order to avoid a bias through including simply a small number of healthy persons who are not representative for the whole population.
The common inclusion criteria for both diagnostic groups were no severe medical disease, free of acute allergies, inflammatory disorders, autoimmune diseases, and clinically apparent infections; those were controlled through extensive medical examinations. Further essential inclusion criteria for schizophrenics contained: (1) being drug-free for at least 3 days, (2) the diagnosis of schizophrenia, (3) no history of psychotropic substance addiction or abuse except nicotine, and (4) no personality disorders according to DSM-IV (American Psychiatric Association, 1994). In addition, normal subjects must be free of any psychiatric disorder and have no first degree biological relative who had ever suffered or been suffering under any psychiatric disease.
Preparations and cytokine measurement
Blood sample of each subject was drawn between 8-9 AM. Two hundreds ml of the drawn whole blood was mixed with 3 ml RPMI 1640 medium (Biochrom; Berlin, Germany) supplemented with 10% of fetal bovine serum and 1% of penicillin streptomycin (Biochrom; Berlin, Germany). Then, a final concentration of 5 mg/ml phytohemagglutinin (PHA) was added to the diluted whole blood. The final mixture of whole blood, medium, and PHA was incubated at 37°C in 5%CO2/95% humidity for 46 hours. After 46 hours, the stimulated whole blood was centrifuged at 6°C by 3270 rpm for 10 minutes; the resultant supernatant was kept at –80°C until analysis. The Human Th1/Th2 Cytokine Cytometric Bead Array Kit-II (CBA: Becton Dickinson Pharmingen, USA) was used to assess Th1/Th2 cytokines including IFN-g, IL-4, and IL-10. Based on the same principles as ELISA, CBA additionally has two big advantages over traditional ELISA; they include that (1) more cytokines can be detected simultaneously with the same amount of test sample and that (2) the detectable rangs are greater than those of ELISA. However, the sensitivities to detect the Th1/Th2 cytokines are comparable to those by ELISA. Therefore, it’s particularly suitable to assess IFN-g/IL-4 and IFN-g/IL-10 ratio due to no inter-assay variance among distinct cytokines by the same individual. Furthermore, the serum levels of prolactin, cortisol, estradiol, testosterone, and the sex-hormone binding globuline (SHBG) were measured by using corresponding Elecsys Kits (Roche Diagnostics; Mannheim, Germany). The measurements of cytokines and hormones were conducted, following the manuals supplied by the manufacturers.
Statistics
The major dependent variables were the IFN-g/IL-4 and IFN-g/IL-10 ratios; additionally, single cytokine IFN-g, IL-4, and IL-10 were likewise compared between schizophrenics and controls. The co-variants included age, prolactin, cortisol, estradiol, testosterone, and SHBG if any of them significantly differentiated schizophrenic patients from normal controls. MANOVA was utilized to evaluate the diversities in distinct hormones. MAN(C)OVA was applied to compare the disparities in Th1/Th2 cytokines and ratios between two different index-groups. The reason to choose MAN(C)OVA, instead of non-parametric significance tests, as the major method to evaluate the data was that MAN(C)OVA enables involvement of more than one dependent variable and co-variation of diverse parameters, whereas a non-parametric test doesn’t. In this study, among the variables of interest (cytokines and hormones) exist reciprocal relationships. A method considering the mutual links among diverse parameters involved such as MANCOVA seems to be more suitable than one without such a possibility to unscramble our question.
Results
Demographical data
The average ages of the whole schizophrenic and control group were evidently different (F = 12.88, p < .001). But the ratios between males and females were undistinguishable between both diagnostic groups (c² = .30, p = .59). If compared the ages of the schizophrenic patients to the normal subjects of their corresponding sex, then only the age diversity between both female diagnostic groups reached a significance level, nevertheless, not that between both male groups (♀SCH vs. ♀CON: F = 12.44, p = .001; ♂SCH vs. ♂CON: F = 2.45, p = .12).
Endocrinological data
The values of all hormones by both schizophrenics and controls are summarized in Table 1. Despite that (1) both genders were mixed in the whole schizophrenic and control group and (2) there was minimal difference in the male/female ratios between both diagnostic groups, comparisons in testosterone and estradiol levels were conducted. The aim to compare testosterone and estradiol levels between the whole schizophrenic and control group was to examine whether any remarkable difference in both sex hormones existed between both diagnostic groups. If there was/were, then it/they would be included into the MANCOVA analysis in order to control its/their effects on Th1/Th2 ratios which were obtained from exactly the same subjects as those of testosterone and estradiol levels. Multi-variance analysis revealed obvious discrepancies in cortisol, prolactin, and SHBG between the whole schizophrenics and normal subjects (cortisol: F = 4.60, p = .03; prolactin: F = 15.20, p < .001; SHBG: F = 3.84, p = .05; total testosterone: F = 2.89, p = .09; estradiol: F = .17; p = .68). The schizophrenic patients had significantly lower cortisol and SHBG, however, higher prolactin levels than normal controls.
If both genders analyzed separately, female patients had markedly reduced cortisol, but enhanced prolactin compared with normal women (♀SCH vs. ♀CON – cortisol: F = 6.09, p = .02; prolactin: F = 7.25, p = .01; estradiol: F = .47, p = .50; total testosterone: F = 1.11, p = .30; SHBG: F = 2.12, p = .15), while male schizophrenics additionally had lower testosterone and SHBG than normal males, apart from the increase in prolactin (♂SCH vs. ♂CON – total testosterone: F = 19.77, p < .001; prolactin: F = 9.37, p = .003; SHBG: F = 6.13, p = .02; cortisol: F = .02, p = .90; estradiol: F = .10, p = .75).
Those clear diversities in age and hormones presented above would be included into the subsequent MAN(C)OVA of the corresponding index-groups.
Table 1
The means (M) and standard deviations (SD) of serum cortisol, prolactin, estradiol, testosterone, and SHBG levels in schizophrenics and healthy controls.
Diagnostic group |
Schizophrenia:M(SD) |
Control: M (SD) |
|||
Hormone |
|
♀ (N = 18) |
♂ (N = 26) |
♀ (N = 35) |
♂ (N = 41) |
Cortisol (mg/L) |
|
160.00 (70.21) |
172.57 (58.25) |
233.86 (116.24) |
174.52 (61.20) |
S |
167.43 (62.93) |
201.85 (94.92) |
|||
Prolactin (ng/ml) |
|
36.65 (47.75) |
28.22 (21.43) |
14.81 (5.80) |
15.74 (11.94) |
S |
31.67 (34.44) |
15.31 (9.56) |
|||
Estradiol (pg/ml) |
|
77.23 (86.09) |
31.94 (8.84) |
62.28 (69.01) |
32.65 (9.21) |
S |
50.47 (59.02) |
46.30 (49.24) |
|||
Testosterone (ng/ml) |
|
.49 (.26) |
4.77 (2.06) |
.73 (.93) |
6.84 (1.73) |
S |
3.02 (2.65) |
4.03 (3.38) |
|||
SHBG (nmol/L) |
|
91.26 (52.67) |
32.90 (13.07) |
116.38 (62.63) |
42.60 (17.04) |
S |
56.77 (45.15) |
76.58 (57.48) |
|||
Th1/Th2 cytokines and ratios in schizophrenia
The whole schizophrenic vs. the whole control group
In general, schizophrenics had lower whole blood Th1/Th2 ratios and produced less cytokines than healthy subjects except IL-4; the outcomes are summarized in Table 2. Nevertheless, MANCOVA demonstrated evident disparities only observed in IFN-g and IFN-g/IL-10 ratio (Fig. 1) if including age, cortisol, prolactin, and SHBG as co-variants since both groups were clearly distinguishable in those regards (IFN-g/IL-10: F = 4.45, p = .04; IFN-g/IL-4: F = .74, p = .39; IFN-g: F = 6.74, p = .008; IL-4: F = .03, p = .86; IL-10: F = .58, p = .45).
Table 2
Comparisons of whole blood cytokine secretions and Th1/Th2 ratios between schizophrenics and healthy subjects.
Whole blood Th1/Th2 cytokine productions and Th1/Th2 ratios [M(SD)] |
||
Group |
Schizophrenia (N =44) |
Controls (N = 76) |
IFN-g (ng/ml) |
30.47 (20.85)** |
45.39 (33.16) |
IL-4 (pg/ml) |
60.61 (47.03) |
54.70 (53.90) |
IL-10 (pg/ml) |
1172.98 (699.56) |
1299.87 (947.15) |
IFN-g/IL-4 |
889.75 (1260.87) |
1285.00 (1427.55) |
IFN-g/IL-10 |
29.80 (20.14)* |
39.72 (26.86) |
Note |
* p £ .05; ** p £ .01. |
|
|
Fig.1: Standardized whole blood IFN-g/IL-4 (IFN/IL4) and IFN-g/IL-10 (IFN/IL10) ratio in the whole schizophrenic and control group (x = extreme value; O = outlier; * p < .05). P = .007** |
The outcomes from the comparisons between both schizophrenic gender subgroups and the corresponding sex of normal subjects are summarized in Table 3.
Female schizophrenics vs. female controls
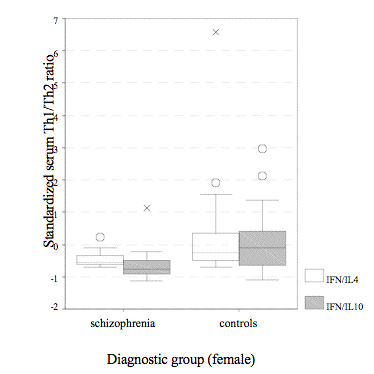
Female patients had lower Th1/Th2 ratios and released less cytokines than normal women except IL-4. Multi-variant comparisons revealed significant diversities between both female subgroups simply found in IFN-g/IL-10 ratio (Fig. 2) and IFN-g if including age, cortisol, and prolactin as co-variants; besides, the disparity in IFN-g/IL-4 (Fig. 2) also showed a trend to be markedly different (♀SCH vs. ♀CON – IFN-g/IL-10: F = 7.86, p = .007; IFN-g/IL-4: F = 3.30, p = .08; IFN-g: F = 5.56, p = .02; IL-4: F = .31, p = .58; IL-10: F = .04, p = .85).
Male schizophrenics vs. male healthy subjects
Generally, male schizophrenics had lower whole blood Th1/Th2 ratios and cytokines than normal men except IL-4. However, between both male diagnostic groups existed no obvious diversity in Th1/Th2 cytokines and ratios according to the results from MANCOVA, regardless of clear differences between both male groups in various hormones (♂SCH vs. ♂CON – IFN-g/IL-4: F = .26, p = .61; IFN-g/IL-10: F = .21, p = .65; IFN-g: F = .09, p = .76; IL-4: F = .71, p = .40; IL-10: F = .08, p = .77).
Table 3
Comparisons of whole blood cytokine levels and Th1/Th2 ratios between schizophrenic (SCH) and control (CON) males/females.
Whole blood Th1/Th2 ratios and cytokines [M(SD)] |
||||
Group |
Female |
Male |
||
Gender |
SCH (N = 18) |
CON (N = 35) |
SCH (N = 26) |
CON (N = 41) |
IFN-g |
23.41 (13.38) ** |
45.22 (32.81) |
35.36 (23.78) |
45.54 (33.86) |
IL-4 |
63.30 (49.37) |
54.85 (38.84) |
58.75 (46.24) |
54.58 (64.55) |
IL-10 |
1368.32 (926.69) |
1397.22 (800.39) |
1037.74 (459.66) |
1216.78 (1059.32) |
IFN/IL4 |
462.03 (310.06) |
1290.69 (1620.74) |
1185.87 (1564.02) |
1280.13 (1260.25) |
IFN/IL10 |
19.64 (12.07)** |
34.77 (21.52) |
36.84 (21.74) |
43.95 (30.31) |
Note |
(1) Unit of IL-4 and IL-10 = pg/ml; unit of IFN-g = ng/ml; (2) IFN/IL4 = IFN-g/IL-4; IFN/IL10 = IFN-g/IL-10; (3) Compared to corresponding sex: ** p £ .01. |
|||
Moreover, due to the possible effects of nicotine use on cytokines (e.g. Fernandez-Real et al., 2003), the influences of nicotine was controlled; no noteworthy impact of nicotine consumption on any whole blood Th1/Th2 cytokine or ratio was found (data not shown).
Conclusion and Discussion
On the whole, the major findings of this study at single cytokine level were in line with most reports in corresponding respects, showing reduced IFN-g, not remarkably enhanced IL-4, and unaltered IL-10 in vitro production by schizophrenics, regardless of their genders (Cazzullo et al., 2002; Kaminska et al., 2001; Wilke et al., 1996).
Schizophrenics as a whole group were found to have a marked Th2-shift. However, if both genders analyzed separately, then Th2-shift was only clearly observed by female patients, but not male schizophrenics. One possible reason for that might be that females generally have higher estradiol, nonetheless, lower testosterone levels than males since estradiol was found to promote Th2 cytokines, while testosterone was shown to inhibit Th2 cytokines (Huber et al., 1999). Nevertheless, if compared schizophrenics to the healthy subjects of corresponding sex, (1) female patients demonstrated an obvious Th2-shift, despite of having similar estradiol levels to normal women, and (2) male schizophrenics did not exhibit an apparent Th2-shift, regardless of having noticeably lower testosterone levels than healthy males. Sex hormones could have contributed to Th1/Th2 imbalance in schizophrenia; however, they were not the only causes since their effects on the Th1/Th2 cytokines/ratios had been excluded. A drawback of this study is that data regarding the menstrual cycle by female subjects were not obtained. However, the inclusion of estradiol as covariant in this study might have compensated this shortage for the reason that the level of estradiol varies with the menstrual cycle (Fass et al., 2000) and it’s rather biological parameters such as estradiol than the menstrual cycle itself has impact on Th1/Th2 balance.
Schizophrenic females additionally had markedly lower cortisol levels than healthy women. Lower cortisol levels in female schizophrenic patients might be the results of chronic stress since in contrast to acute stress, chronic stress was found to induce reduced cortisol levels through down-regulating glucocorticoid receptor (Mizoguchi et al., 2001). Cortisol in humans can act not just as stress hormones. It was newly shown to play an essential role in maintaining prefrontal cortical cognitive function (Mizoguchi et al., 2004). Regardless of the role of cortisol in the cognitive function, cortisol seemed not to be the key reason for the Th2-shift in female schizophrenics because it was reported to induce a shift from Th1 to Th2 (Visser et al., 1998; Franchimont et al., 1998). Despite of having lower cortisol levels, a significant Th2-shift was observed in female subjects with schizophrenia. Moreover, female patients were much older than healthy women. An age-related shift towards Th2 was lately described (Sandmand et al., 2002; Karanfilov et al., 1999). Nevertheless, after including age as co-variant, schizophrenic women still revealed a clear Th2-shift.
In addition, both male and female schizophrenic patients showed tremendously higher prolactin levels than healthy controls of the same sex. Highly enhanced prolactin levels in patients might be raised by factors like anti-psychotic medication and/or stress (Hummer and Huber, 2004; Rodriguez et al., 2003). Nevertheless, no matter which source(s) resulted in augmented prolactin levels by schizophrenics, enhanced prolactin could have re-directed Th2 to Th1 by male schizophrenics since prolactin was found to amplify IFN-g(Matalka, 2003; Breidthardt et al., 2002); so Th2-shfit was less profound by male patients. However, female schizophrenics, regardless of highly increased prolactin levels, showed a marked Th2-shift. Even if the impacts of prolactin on IFN-g is not montonic as a few studies reported, higher concentration of prolactin likely led to rather decreases than increases of IL-2-induced IFN-g synthesis (Matera and Mori, 2000). A Th2-shift remained apparent by female schizophrenics after co-varying with the levels of prolactin. In this study, data regarding wash-out-periods by schizophrenic patients were not complete. Nevertheless, biological parameters such as prolactin and testosterone were measured. The goal to collect information about “wash-out-periods” in schizophrenia was to examine the effects of diverse neuroleptics on Th1/Th2 ratios. As a matter of fact, it’s rather biological variables such as prolactin and testosterone than a clinical parameter like “wash-out-period” having direct impacts on Th1/Th2 ratios. Therefore, the inclusion of prolactin and testosterone into the analysis as co-variants in our study should have compensated the shortage of not having entire data concerning “wash-out period”.
Furthermore, it’s noteworthy that Th2-shift in the whole blood in vitro system does not mean an obligatory Th2-shift in serum in vivo system since (1) markedly more components of the cytokine network are involved in vivo than in vitro, (2) influencing factors from the endocrine system such as diverse hormones react to stimuli in the whole blood system not dynamically as they do in vivo, and (3) mitogen effects could be different from those raised by stimuli such as viruses in vivo. For example, IL-4-mediated mitogenic effects involve activation of IL-4-induced phosphotyrosine substrate, while IL-4-specific gene induction involved STAT6 (Quelle et al., 1995; Hou et al., 1994). However, whole blood Th2-shift in schizophrenia could indicate deficits at another level. Probably, the latter reason also explains why the reduction in whole blood IFN-g/IL-4 by schizophrenics was less noticeable than that in IFN-g/IL-10 ratio.
So, in sum, this study demonstrates that female schizophrenics, but not male patients, showed a clear Th2-shift in whole blood after 46-hour-PHA-stimulation. The Th2-shift in female patients appeared to be not the effects of stress hormones such as cortisol and prolactin or those of sex hormones including testosterone and estradiol since Th2-shift remained remarkable after the impacts of those hormones stated above were controlled. Th2-shift in female schizophrenic patients seems to be rather a consequence of disease process.
Acknowledgments
This study was supported by the Theodore Vada Stanley Research Institute and the Hans-Seidel Foundation.
References
Agnello,D., Lankford,C.S., Bream,J., Morinobu,A., Gadina,M., O'Shea,J.J., and Frucht,D.M., 2003 Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol 23, 147-161.
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition., American Psychiatric Association, ed., Washington, DC: American Psychiatric Association).
Angele,M.K., Knoferl,M.W., Ayala,A., Bland,K.I., and Chaudry,I.H., 2001 Testosterone and estrogen differently effect Th1 and Th2 cytokine release following trauma-haemorrhage. Cytokine 16, 22-30.
Arolt,V., Rothermundt,M., Wandinger,K.P., and Kirchner,H., 2000 Decreased in vitro production of interferon-gamma and interleukin-2 in whole blood of patients with schizophrenia during treatment. Mol Psychiatry 5, 150-158.
Breidthardt,T., Frohn,C., Luhm,J., Kirchner,H., and Brand,J.M., 2002 Prolactin induces enhanced interferon gamma release in peripheral whole blood after stimulation with either PHA or LPS. Immunobiology 206, 424-431.
Cazzullo,C.L., Sacchetti,E., Galluzzo,A., Panariello,A., Adorni,A., Pegoraro,M., Bosis,S., Colombo,F., Trabattoni,D., Zagliani,A., and Clerici,M., 2002 Cytokine profiles in schizophrenic patients treated with risperidone: a 3-month follow-up study. Prog Neuropsychopharmacol Biol Psychiatry 26, 33-39.
Cazzullo,C.L., Scarone,S., Grassi,B., Vismara,C., Trabattoni,D., Clerici,M., and Clerici,M., 1998 Cytokines production in chronic schizophrenia patients with or without paranoid behaviour. Prog Neuropsychopharmacol Biol Psychiatry 22, 947-957.
Dimitrov,S., Lange,T., Fehm,H.L., and Born,J., 2004 A regulatory role of prolactin, growth hormone, and corticosteroids for human T-cell production of cytokines. Brain Behav Immun 18, 368-374.
Elenkov,I.J. and Chrousos,G.P., 2002 Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci 966, 290-303.
Franchimont,D., Louis,E., Dewe,W., Martens,H., Vrindts-Gevaert,Y., De Groote,D., Belaiche,J., and Geenen,V., 1998 Effects of dexamethasone on the profile of cytokine secretion in human whole blood cell cultures. Regul. Pept. 73, 59-65.
Hou,J., Schindler,U., Henzel,W.J., Ho,T.C., Brasseur,M., and McKnight,S.L., 1994 An interleukin-4-induced transcription factor: IL-4 Stat. Science 265, 1701-1706.
Huber,S.A., Kupperman,J., and Newell,M.K., 1999 Estradiol prevents and testosterone promotes Fas-dependent apoptosis in CD4+ Th2 cells by altering Bcl 2 expression. Lupus 8, 384-387.
Hummer,M. and Huber,J., 2004 Hyperprolactinaemia and antipsychotic therapy in schizophrenia. Curr Med Res. Opin 20, 189-197.
Kaminska,T., Wysocka,A., Marmurowska-Michalowska,H., Dubas-Slemp,H., and Kandefer-Szerszen,M., 2001 Investigation of serum cytokine levels and cytokine production in whole blood cultures of paranoid schizophrenic patients. Arch Immunol Ther Exp (Warsz) 49, 439-445.
Karanfilov,C.I., Liu,B., Fox,C.C., Lakshmanan,R.R., and Whisler,R.L., 1999 Age-related defects in Th1 and Th2 cytokine production by human T cells can be dissociated from altered frequencies of CD45RA+ and CD45RO+ T cell subsets. Mech. Ageing Dev. 109, 97-112.
Matalka,K.Z., 2003 Prolactin enhances production of interferon-gamma, interleukin-12, and interleukin-10, but not of tumor necrosis factor-alpha, in a stimulus-specific manner. Cytokine 21, 187-194.
Matera,L. and Mori,M., 2000 Cooperation of pituitary hormone prolactin with interleukin-2 and interleukin-12 on production of interferon-gamma by natural killer and T cells. Ann N Y Acad Sci 917, 505-513.
McGuirk,P. and Mills,K.H., 2002 Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol 23, 450-455.
Miyaura,H. and Iwata,M., 2002 Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol 168, 1087-1094.
Mizoguchi,K., Ishige,A., Takeda,S., Aburada,M., and Tabira,T., 2004 Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J Neurosci. 24, 5492-5499.
Mizoguchi,K., Yuzurihara,M., Ishige,A., Sasaki,H., Chui,D.H., and Tabira,T., 2001Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology 26, 443-459.
Muller,N., Riedel,M., Gruber,R., Ackenheil,M., and Schwarz,M.J., 2000 The immune system and schizophrenia. An integrative view. Ann N Y Acad Sci 917, 456-467.
Muller,N., Ulmschneider,M., Scheppach,C., Schwarz,M.J., Ackenheil,M., Moller,H.J., Gruber,R., and Riedel,M., 2004 COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci. 254, 14-22.
Quelle,F.W., Shimoda,K., Thierfelder,W., Fischer,C., Kim,A., Ruben,S.M., Cleveland,J.L., Pierce,J.H., Keegan,A.D., Nelms,K., and ., 1995 Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol 15, 3336-3343.
Rodriguez,T., Albuquerque-Araujo,W.I., Reis,L.C., Antunes-Rodrigues,J., and Ramalho,M.J., 2003 Hypothyroidism attenuates stress-induced prolactin and corticosterone release in septic rats. Exp Physiol 88, 755-760.
Rothermundt,M., Arolt,V., Leadbeater,J., Peters,M., Rudolf,S., and Kirchner,H., 2000 Cytokine production in unmedicated and treated schizophrenic patients. Neuroreport 11, 3385-3388.
Rothermundt,M., Arolt,V., Weitzsch,C., Eckhoff,D., and Kirchner,H., 1998 Immunological dysfunction in schizophrenia: a systematic approach. Neuropsychobiology 37, 186-193.
Sandmand,M., Bruunsgaard,H., Kemp,K., Andersen-Ranberg,K., Pedersen,A.N., Skinhoj,P., and Pedersen,B.K., 2002 Is ageing associated with a shift in the balance between Type 1 and Type 2 cytokines in humans? Clin Exp Immunol 127, 107-114.
Schwarz,M.J., Chiang,S., Muller,N., and Ackenheil,M., 2001a T-helper-1 and T-helper-2 responses in psychiatric disorders. Brain Behav Immun 15, 340-370.
Schwarz,M.J., Muller,N., Riedel,M., and Ackenheil,M., 2001b The Th2-hypothesis of schizophrenia: a strategy to identify a subgroup of schizophrenia caused by immune mechanisms. Med Hypotheses 56, 483-486.
Visser,J., Boxel-Dezaire,A., Methorst,D., Brunt,T., de Kloet,E.R., and Nagelkerken,L., 1998 Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood 91, 4255-4264.
Wilke,I., Arolt,V., Rothermundt,M., Weitzsch,C., Hornberg,M., and Kirchner,H., 1996 Investigations of cytokine production in whole blood cultures of paranoid and residual schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 246, 279-284.
Xie,H.F., Li,J., and Shi,W., 2002 [Effect of corticosteroids on the balance of Th cytokines in patients with systemic lupus erythematosus]. Hunan. Yi. Ke. Da. Xue. Xue. Bao. 27, 533-535.
Zhang,X.Y., Zhou,D.F., Cao,L.Y., Zhang,P.Y., and Wu,G.Y., 2002 Decreased production of interleukin-2 (IL-2), IL-2 secreting cells and CD4+ cells in medication-free patients with schizophrenia. J Psychiatr Res. 36, 331-336.
Authors:
Sonnig Sue Whei Chiang, Michael Riedel, Norbert Müller,
Manfred Ackenheil, Rudolf Gruber, Markus Schwarz
Psychiatric Hospital of Munich University
Neurobiochemistry and Psychopharmacology Section
Nussbaumstr. 7, 80336 Munich, Germany
Corresponding author: Sonnig Sue Whei Chiang
Neurobiochemistry and Psychopharmacology Section
Psychiatric Clinic of Munich University
Tel.: +49 89 5160 3381, Fax: +49 89 5160 4741
Key words: Th2-shift, schizophrenia, cytokine, hormones, whole-blood-assay.
This draft December 23, 2004
Abstract word count 159
Manuscript word count: 4445
Acknowledgments
This study was supported by the Theodore-Vada-Stanley-Research-Institute and the Hans-Seidel-Foundation.
First Published 28th December 2004
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-



