Browse through our Journals...
In-vitro permeation of repaglinide from polymeric membrane systems across human cadaver system
G.D. Gupta, Parul Patel*, Amit Chaudhary, Y.S. Tanwar
* corresponding author:
B.N. College of pharmacy, Udaipur 313 002 (Rajasthan) INDIA
Abstract:
Prepared matrices were evaluated for their physico-chemical characteristics: weight variation, thickness uniformity, tensile strength and percent elongation at break, drug content uniformity, , in-vitro permeation study, interaction studies and stability studies. In-vitro permeation profile across human cadaver skins using a modified Keshery- Chien (K-C) diffusion cell were reported. The permeation rate was enhanced. Different models were applied to evaluate release mechanism and kinetics. Criteria for selecting the most appropriate model was based on goodness of fit and F-statistics. Based on the best fit of permeation data to different mathematical models it was concluded that all the formulations followed zero order kinetics with matrix diffusion pattern. The permeation rate was enhanced which was confirmed by skin flux, enhancement factor and permeability coefficient.
1. Introduction
In the last decades, transdermal dosage forms have been introduced for providing controlled delivery via skin into the systemic circulation (Tymes et al., 1990). TDD systems has many advantages over conventional modes of drug administration, especially, it avoids hepatic first pass metabolism and improves patient compliance (Codes et al., 1987). Intensive research has showed that transdermal route is potential mode of delivery of lipophillic drugs in the systemic circulation (Schaefer et al., 1982). Matrix based transdermal formulations have been developed for a number of drugs such as nitroglycerine (Pai et al., 1994).
Repaglinide is a lipophillic drug used for lowering the blood glucose level by stimulating the insulin secretion. After oral administration peak plasma concentrations of repaglinide is reeched within one hour. It possesses low oral bioavailability (56%) due to hepatic first pass metabolism after oral administration and has a short biological half-life of ~1h (Hardman et al., 2001). Which makes frequent dosing necessary to maintain the drug within the therapeutic blood level for long periods.
Among the various methods of permeation enhancement, use of permeation enhancers are the most convenient and relatively strong effects.
Our aim was to develop a matrix type monolithic transdermal system for Repaglinide and to investigate the effect of permeation enhancers on the increase in the rate and amount of drug permetion across the skin.
2. Materials and methods
2.1. Materials
Repaglinide was procured from Sun Pharmaceuticals Ltd., Silvassa (INDIA), Eudragit NE 30D (Ed NE) was obtained from S. Zavery& Co., Mumbai as a gift sample, human cadaver skin from the chest region was obtained after post-mortem of males in the age group 20-6- years from Seth V.S. General Hospital Ahmedabad, A K-C diffusion cell with a volume of 75 ml and diffusional area of 4.906 cm2 were employed. Dibutyl phthlate (DBP), acetone, methanol, propylene glycol (PG), oleic acid (OA), tween-60, span-80 and isopropyl myristate (IPM) were of analytical grade.
2.2. Methods
- Casting of matrices
The repaglinide- Ed NE matrices were prepared by a casting process. About 500 mg of crushed pieces of dried films of Ed NE suspension and drug were dissolved in 10 ml of acetone. Hydrophilic additive PG at a concentration of 15% w/w was dropped into drug containing polymer solution with mixing by continuous stirring. This method was chosen in order to produce large undamaged pieces of membrane with no orientation of molecules (Bodmeier et al., 1987). In formulations B-2, B-3, B-4 and B-5 15% w/w based on polymer weight OA, Tween-60, Span-80 and IPM respectively. Formulation B-1 was prepared without permeation enhancer.
For casting of the matrices aluminium foil was spread on glass petridishes. Rate of evaporation was controlled by inverting a funnel on it. The matrices were then removed, packed in aluminium foil and stored in dessicator at room temperature for further studies.
2.2.2. Evaluation of matrices
2.2.2.1. Physico-chemical properties
Uniformity of weight was determined by weighing five matrices of 4.906 cm2 (Jain et al., 1996).
Assessment of thickness was done on three patches the mean and standard deviation were calculated (Nafee et al., 2003).
The tensile strength and percent elongation at break of the films was measured using tensile strength instrument (Allen et al., 1972).
2.2.2.2. In-vitro diffusion study
Matrices of Repaglinide measuring 4.906 cm2 were subjected to in-vitro diffusion testing using a K-C diffusion cell (Keshary et al., 1984). Cadaver human skin of 20-60 yr old male from Seth V.S. General Hospital, Ahmedabad. The separated epidermal layer was used as such for the skin permeation study.
The epidermis was clamped between the donar and recipient compartments, the matrix was placed in a donar compartment over the skin. The receiver phase was 30% v/v methanolic isotonic phosphate buffer (IPB) pH 7.4 stirred on a magnetic stirrer. Methanol was used as a co-solvent for the drug in the buffer. The whole assembly was kept in a water bath maintained at 37± 1° C. The amount of drug diffused through cadaver human skin was determined by removing 5 ml samples at predetermined time intervals and the same volume was replaced by pre-warmed 30 % v/v methanolic IPB (37± 1° C). The samples were analysed after suitable dilutions at 239 nm using a thermospectronic UV-Vis spectrophotometer.
2.2.2.3. Effects of an enhancer on the permeation of Repaglinidethrough the human cadaver skin
The enhancers were used to affect the fluidity of the stratum corneum structure and the drug could then permeate easily through the skin, defined as the enhancement factor (Shin et al., 2002)
![]() EF = Drug flux from matrix containing permeation enhancer
EF = Drug flux from matrix containing permeation enhancer
Drug flux from matrix without permeation enhancer
- Results and Discussions
3.1. Physico-chemical properties
The matrices of Repaglinide showed satisfactory physico-chemical properties. Weight variation varies from 32.13 to 33.60 mg. the thickness of the films varies from 0.132 to 0.165 mm. the formulation B-1 shows least percentage of elongation at break, whereas B-3 shows higher percent of elongation. The result indicates that the thickness is directly proportional to the tensile strength.
3.2. In-vitro permeation profile
Permeation of Repaglinide across cadaver human skin for different formulations is shown in Fig. 1. The permeation profile followed zero order kinetics. The permeability flux and permeability coefficient for different formulations are shown in Table 2 along with the r2 values which shows the straightness of the plot between cumulative percent drug permeated vs. time. Plot of cumulative % drug permeated vs. square root of time indicates that all the formulations followed Higuchian matrix diffusion pattern.
3.3. Effect of enhancers on permeation of Repaglinide through the human cadaver skin.
The effect of enhancers such as OA, Tween-60, Span-80, IPM on the transport of Repaglinide through the skin was investigated at a concentration of 15%w/w. Permeation enhancing effects were evaluated by an enhancement factor. Table 2 shows the permeation data of Repaglinide with and without enhancers. The permeation of drug from the Ed NE matrix containing enhancers through human skin shoiwed better enhancing effect, among enhancers used, Span-80 showed the best enhancement.
Conclusions
For controlling the delivery of Repaglinide, the Ed NE matrix containing permeation enhancer would be a favourable development.
Acknowledgements
The authors are grateful to Dr. A.C Rana, Director and Principal of B.N College of pharmacy, Udaipur (Rajasthan) for providing the facilities for this work. The gift sample of Propranolol hydrochloride by Sarabhai chemicals Ltd, Vadodara (India) is highly acknowledged.
References
Allen, D.J., DeMacro, J.D. and Kwan, K.C., 1972. Free films I: apparatus and preliminary evaluation. J. Pharm. Sci., 61(1) 100-109.
Aquil, M. and Asgar, A., 2002. Monolithic matrix type transdermal drug delivery systems of pinacidil monohydrate: in vitro characterization. Eur. J. Pharm. Biopharm., 54, 161-164.
Bodmeier, R., Paeratakul, O., 1989. Evaluation of drug containing polymeric films prepared from aqueous latexes. Pharm. Res., 61, 725-730.
Code, G., Fischer, W., Legler, U., Wolff, H.M., 1987. Proceedings of 3rd TTS symposium, Tokyo, pp. 3-8.
Hardman, J.G, Limbird, L.E. 2001. In: Goodman and Gillman’s (Ed.), The Pharmacological Basis of Therapeutics, 10th Edn., Mac Grawhill, New York, pp. 1704-5, 2002.
Keshary, P.R., Chien, Y.W. 1984. Mechanisms of transdermal controlled nitroglycerin administration (1): development of finite-dosing skin permeation system. Drug Dev. Ind. Pharm., 10, 883-913.
Nafee, N.A., Boraie, N.A., Ismail, F.A., Mortada, L.M., 2003. Design and characterization of mucoadhesive buccal patches containing cetylpyridinium chloride. Acta. Pharm., 53, 199-212.
Pai, R.M., Desai, M.S., Babtiwale, A.D., 1994. Adhesive matrix type transdermal drug delivery system for Nitroglycerine. Drug Dev. Ind. Pharm., 20, 1905-1909.
Schaefer, H., Zesch, A., Stuttgen, G., 1982. Skin permeability, Springer-Verlag, New York, pp. 123-146.
Shin, S.-C., Lee, H.-J., 2002. Enhanced transdermal delivery of triprolidine from the ethylene-vinyl acetate matrix. Eur. J. Pharm. Biopharm., 54, 325-328.
Tymes, N.W., Shah, V.P., Skelly, J.P. 1990. In vitro profile of estradiol transdermal therapeutic systems. J. Pharm. Sci., 79,601-602.
Table 1
Composition of different formulations
Formulations B-1 B-2 B-3 B-4 B-5 Ingredients Eudragit NE 30 D (% w/v) 5 5 5 5 5 Oleic acid (%w/w)* - 15 - - - Tween-60 (%w/w)* - - 15 - - Span-80 (%w/w)* - - - - - IPM (%w/w)* - - - - 15 |
* based on polymer weight
Table 2
Effect of permeation enhancers on the flux of Repaglinide from Eudragit NE 30D matrix
Formulations B-1 B-2 B-3 B-4 B-5 In vitro parameters Cumulative % of the drug permeated in 24 h 63.49 70.09 77.87 88.50 74.00 R2 24 h 0.9922 0.9859 0.9658 0.9499 0.9770 Flux (mg/cm2/hr x 10-2) 4.562 4.917 5.595 6.359 5.116 Permeability coefficient (cm/hr x 10-2) 2.64 2.92 3.24 3.68 3.08 Enhancement Factor 1.18 1.27 1.45 1.64 1.32 |
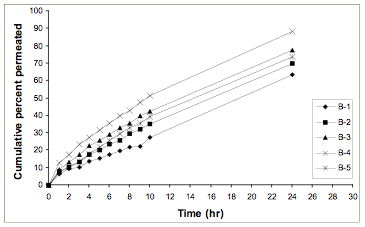
Fig. 1. Permeation of Repaglinide across cadaver human skin

Fig. 2. Plots of cumulative percent drug permeated versus square root of time
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


