The permeation profile of drug from both patches HE2 and PVA10 when plotted,between permeation data against square root of time shows linear relationship indicating drug permeation followed Higuhi equation (Fig.2).
Saraf Swarnlata*, Saraf S1, and Dixit V.K. 2
1Institute of Pharmacy, Pt. Ravishankar Shukla University, Raipur (CG) 492 010
2Department of Pharmaceutical Sciences, Dr. H.S. Gour V.V., Sagar, M.P.
*e-mail: swarnlata_saraf@rediffmail.com
Summary
Transdermal delivery of timolol meliate was tried for both reservoir as well
as matrix system. The physically stable patches regarding drug contents, tensile
strength, toughness and WVT were found for PVA10 and HE2 formulation. Both patches
follows diffusion controlled drug permeation and high permeation with PVA10
while reservoir system follows zero order permeation kinetics.
Introduction
Timolol maleate is a beta adrenoceptor-blocking agent used in treatment of cardiovascular
diseases like myocardial infarction, angina pectoris, hypertension, respiratory
complications and migraine. It is 8-10 times potent than propranolol 1. The
main limitation of therapeutic effectiveness of timolol maleate is its higher
frequency of drug dosing and short biological half-life, high first pass metabolism
and poor bioavailability by oral route. . It is rapidly absorbed from gastrointestinal
tract with peak plasma concentration of 5-10 ng/ ml after 1 hr2, it is metabolized
up to 80% in liver with a mean half-life of 2.0-2.5 hr.3, thus necessitating
frequent administration of larger doses to maintain therapeutic drug level.
Therefore, To maintain effective plasma concentration and to avoid sub therapeutic
and toxic concentration, a continuous delivery of timolol maleate is required.
The transdermal route is, therefore, a better alternative, to achieve constant
plasma level, which additionally warrants less frequent dose regime.
The present study has been selected transdermal delivery system to achieve maximum
therapeutic benefit.
MATERIALS AND METHODS
Gift sample of timolol maleate (TM) was obtained from Cadila Antibiotics Ltd.,
Ahamdabad. Hydroxy propyl methylcellulose was procured from Warner Hindustan
Ltd., Hyderabad. Dibutyl phthalate, ethyl cellulose, polyvinyl alcohol (PVA),
HEPES buffer, procured from Sigma Chemical Co. St. Locus Mo, USA. All other
chemicals and regent used were of analytical reagent grade.
Fabrication of matrix diffusion patches
Matrix patches were casted4 on mercury using stainless steel rings having inner
diameter of 1.5 cm and thickness 0.5 cm were used for holding the polymer solution
on mercury surface. Two type of polymer patches were prepared; HPMC and EC combination
and with PVA. The polymer solutions were prepared by dissolving HPMC 10% ands
EC 10 % separately in methanol- chloroform (1:1) mixture. Both solutions were
mixed together in combination of 1:9, 2:8, 3:7, 4:6 and 5:5 respectively using
1% dibutyl phthalate as plasticizer. A weighed amount of drug is dispersed in
polymer mixture then poured in ring placed on mercury surface in petri dish
at a uniform place and solvent evaporation was controlled by conveying with
funnel. After evaporation of the solvent, the film was taken out from the metal
ring by sharp knife and preserve in aluminum foil. Similar procedure was adopted
to prepare PVA matrix patch preparation having polymer concentration of 5, 10.and
15% in water with 0.5% glycerin as plasticizer.
Physical characterization
Thickness of polymeric patch was measured by using a dial gauge (Mercer, England),
having least count of 0.002 mm. To maintain its shape, enough hardness is required
to resist independence or penetration was determined by Hall’s method4
and calculated as a functional weight. In order to determine the elongation
as a tensile strength, the polymeric patch was pulled by means of a pulley system;
weights were gradually added to the pan to increase the pulling force till the
patch was broken. The elongation i.e. the distance traveled by the pointer before
break of the patch was noted with the help of magnifying glass on the graph
paper5, the tensile strength was calculated as kg/ cm2. The presence of moisture
may not affect the hardness of patch in the normal environmental conditions,
but it may affect in exaggerated conditions. The polymer patches were cut, weighed
and placed in a humidity chamber maintained at 68% RH for 72 h for equilibrium.
After 72 h polymer patch were taken out and weight accurately. The difference
between initial and final weight was computed as percentage moisture absorbed.
The water vapor transmission (WVT) from the film was calculated by Crowfold
and Esmeric formula 6. It was determined at 25+ 20C at 68%RH. Glass weighing
bottles of equal diameter (2.5 cm) and height (5.0 cm) were used as WVT cells.
A weight quantity of anhydrous calcium chloride was taken up to 10 mm height
in each cell and a thin layer of silicon adhesive grease applied over the brim
then patch was placed on brim and adhesive was allowed to set for 5 minutes.
The cells are accurately weighed and kept in humidity chamber maintained at
68% RH for 24 h. After 24 h, the cells were again weighed and an increase in
weight was considered as a quantitative measure of the moisture transmitted
through the patch. Drug distribution studies: The distribution of drug in polymer
patch effect the release rate. It was studied microscopically with the help
of Lietz Microscope to observe uniform distribution of drug in patch.
Stability studies:
Stability studies of all patches were performed at different storage condition
by measuring tensile strength, moisture content and drug content (spectrophotometric
method). The measurement were carried out by keeping the patches at different
conditions of temperature 280C, 350C and 500C and relative humidity of 30%,
50%, 68 % for storage period of three months at room temperature (25±10C).
The patches, which maintained uniformity, shape, toughness, drug content and
flexibility at all temperature and RH, were selected for further permeation
studies.
Skin Preparation:
The full thickness human cadaver skin was washed with purified water after removal
of all subcutaneous fat and hairs and cut in to pieces for experimental use.
The skin pieces were soaked in HEPES buffer and store in freezer at 300C until
used. Just before the experiment, it was thawed at room temperature and checked
for any microscopical damage.
In vitro drug permeation studies:
A drug permeation study was carried out with drug solution in HEPES buffer pH7.4
and stable patches through human cadaver skin using modified kieshry- chein
diffusion cells. The concentration of drug kept similar in drug solution in
HEPES buffer and patches to compare permeation profile. The Patches and drug
solution were kept on stratum cornium side of cells and this patch - skin -
complex sandwiched between donor and receptor compartment. The receiving compartment
contains blank 10.0 ml of HEPES buffer pH 7.4 and touches the dermal side of
the skin. The whole of the assembly was kept on magnetic stirrer, which thermostatically
controlled at 37+ 2oC at 100 rpm. Samples were withdrawn at pre set time interval
from the receiving compartment and analyzed spectrophotometrically at 294 nm
using shimadzu-1601 UV- visible spectrophotometer. The fresh buffer in receiving
compartment was replaced after each withdrawal. The permeation studies determined
for period of 4 h. and calculated as cumulative percent drug permeated.
Results
In present studies, the polymer and plasticizer choice for patch preparation
were based on no interaction with drug and HEPES buffer with considerable stability.
Various combinations of polymers hydroxy propyl methyl cellulose (HPMC) and
ethyl cellulose (EC) and various concentration of polyvinyl alcohol were tried
for patch preparation and evaluated for physical studies (Table 1).
| Formulation Code | Polymer Used | Polymer Core % w/v | Thickness Mm |
Hardness Gm/cm |
Tensile strength Kg/cm2 |
WVT gm/cm2 / 4 h |
Moisture absorption % |
| HE1 | HPMC:EC | 1:9 | 0.034 |
322 |
2.12 |
2.20 |
2.11 |
| HE2 | HPMC:EC | 2:8 | 0.036 | 314 |
2.98 |
2.63 |
2.43 |
| HE3 | HPMC:EC | 3:7 | 0.035 | 312 |
3.00 |
2.98 |
2.88 |
| HE4 | HPMC:EC | 4:6 | 0.035 | 306 |
3.51 |
3.23 |
3.03 |
| HE5 | HPMC:EC | 5:5 | 0.033 | 302 |
3.83 |
3.40 |
3.1 |
| P5 | PVA | 5.0 | 0.031 | 290 |
2.00 |
3.02 |
2.99 |
| P10 | PVA | 10 | 0.036 | 298 |
2.10 |
0.32 |
3.22 |
| P15 | PVA | 15 | 0.034 | 302 | 2.54 | 4.01 | 4.00 |
Table 1: Physical evaluation of polymeric patch of Timilol maleate
The main physical evaluations considered for stability were WVT rate, tensile strength and % moisture absorption and drug content. The polymer patch prepared with combination in the ratio of 2:8 HPMC and EC (HE2), PVA of 10% w/v (P10) were found to be satisfactory at all temperatures and relative humidity. On the basis of physical and stability studies, the patches HE2 and PVA10 were considered for permeation studies. The in- vitro permeation studies of drug solution and patches were performed to observe permeation profile. The permeation profile of both solution and patches were compared
Discussion
The in- vitro stability studies of all patches were performed in terms of stability
against storage conditions and aging effect, because TM is a drug used for long
therapy to maintain its therapeutic effect especially when used as antihypertensive
agent. The physical characterization like tensile strength, moisture absorption
and WVT rate and drug content were main parameter for stability studies, which
revealed no appreciable changes occurred in patches (HE2 & PVA10) at normal
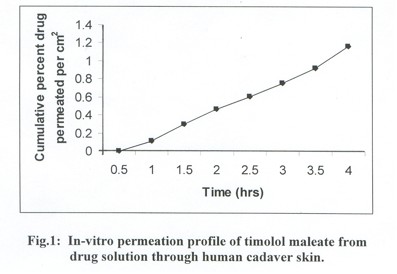
storage conditions (250C+1). The drug solution permeation profile suggests that
it followed Fick’s law of diffusion. Linear relationship between cumulative
percent drug permeated verses time indicate zero order permeation of drug through
human cadaver skin with lag time 35 minutes. The transdermal permeability coefficient
of TM was calculated to be 323x10-5 +0.02cm2/h from the steady state portion
of the curve (Fig.1).

The permeation profile of drug from both patches HE2 and PVA10 when plotted,between
permeation data against square root of time shows linear relationship indicating
drug permeation followed Higuhi equation (Fig.2).
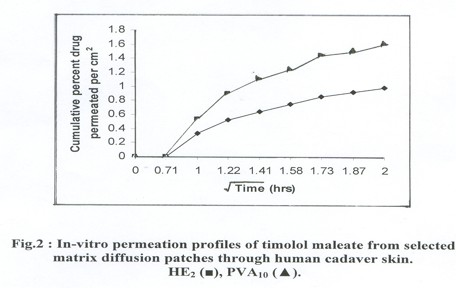
The permeation profile
data of patches were plotted in log values with time, the slope value comes
near to 0.49+0.01SD (~ 0.5), suggesting drug permeation is controlled by diffusion
within the matrix rather than by skin.
When we compare both patches, the PVA10 system provide higher 1.589 + 0.20 SD
% drug/ cm2 of permeation rather than HE2, i.e., 0.987 +0.20 % drug/ cm2 in
4h of period.
Conclusion
The studies suggest that
both reservoir as well as matrix system of transdermal delivery of TM is possible.
The reservoir system followed zero order while the matrix system followed first
order release profile. Among both matrix systems PVA10 patch have more permeability
than HE2 patch.
References
1. Napolian, L. A., Smith, R. L., Proceed. Int. Symp, Controlled Release Bioact.
Mater., 17, Controlled Released Society Inc. USA 1990, Abst. No. D220.
2. Remington Pharmaceutical Sciences, 18th Ed., Mark Publishing Company, Pennsylvania,
1990, P 1676.
3. Vermeji P. J,. Pharm. Pharmacol, 1978, 50,53.
4. Sciarria, J. J., Patel, S. P., J. Pharm. Sci., 1975, 64, 128.
5. Allen, D. J., DeHerco J. D., Kwan, K. C., J. Pharm. Sci., 1972, 61, 107.
6. Crawford, R. R., Esmerian, O. K., J. Pharm. Sci., 1971, 60, 314.
First Published February
4th 2006
All pages copyright ©Priory Lodge Education Ltd 1994-2006.