A STUDY ON THE EFFECT OF DIFFERENT ACRYLIC POLYMERS ON FRUSEMIDE LOADED CALCIUM ALGINATE MICROPELLETS PREPARED BY IONOTROPIC GELATION TECHNIQUE
AMITAVA GHOSH *, L.K NATH1 AND PARTHA ROY
Himalayan Pharmacy Institute, Majitar, East Sikkim- 737136
1 Professor, Dept. Of Pharmaceutical Sciences, Dibrugarh University, Assam
ABSTRACT
The objective of this study is to encapsulate drugs in different acrylic polymers of varying solubility in an absolute aqueous environment. The micropellets were prepared using ionotropic gelation technique, where gelation of anionic sodium alginate, the primary polymer, was achieved with oppositely charged counterion to form microparticles which were further made sustained by using different acrylic polymers namely Acrycoat E30D (poly [ethyl acrylate methyl methacrylate]), Acrycoat L30D (poly [ethyl acrylate methyl methacrylate]), Acrycoat S100 (poly [ethyl acrylate methyl methacrylate]). The effect of these polymers on the release profile of the drug has been reported in this paper. Frusemide, a potent diuretic, was selected as the model drug for the experiments.
Nine set of formulations were prepared using Acrycoat E30D (E1, E2, E4); Acrycoat L30D (L1, L2, L4) and Acrycoat S100 (S1, S2, S4) at concentration (1%, 2%, 4%w/w). The final formulations were subjected to several characterization studies. All the batches sustained the release of the drug for more than 8 hours. Among all acrylic colloidal polymer dispersion, Acrycoat E30D showed high encapsulation efficiencies and maximum prolongation of drug release.
KEYWORDS- Micropellets, Ionotropic gelation, Frusemide, Acrylic polymers
INTRODUCTION
One of the common methods of controlling the rate of drug release is microencapsulation. The encapsulation techniques (e.g., solvent evaporation or coacervation–phase separation) normally involve water insoluble polymers as carriers which require large quantity of organic solvents for their solubilization. (Bodmeier R, et al 1991)(Baken JA. 1987) As a result the processes become vulnerable to safety hazards, toxicity and increases the cost of production making the techniques non reproducible, economically and ecologically at an industrial scale. These concerns demand a technique free from any organic solvent. Thus, the objective of this study is to encapsulate drugs of varying solubility within water insoluble acrylic polymers in an absolute aqueous environment.
Recently, aqueous polymeric dispersions have played a great role in replacing organic solvents in the coating of solid dosage forms with water soluble polymers.( Lehmann K. 1989)( Steuernagel CR. 1989)( Bodmeier R.et al1993) These polymeric dispersions forms a homogenous film(JamesW.McGinity.1997) on drying and provides a diffusion controlled release of the drug from the polymer matrix.
The micropellets were prepared using ionotropic gelation technique (Lim F. et al 1980) ( Segi N. et al 1989)( Bodmeier R.et al 1989) where gelation of anionic polysaccharide sodium alginate, the primary polymer of natural origin, was achieved with oppositely charged calcium ions, acting as counterion,( Lim LY.et al 1997) to form instantaneous microparticles. The micropellets thus produced were further made sustained by using different polymers namely Acrycoat E30D (poly [ethyl acrylate methylmethacrylate]) – a synthetic water insoluble aqueous polymeric dispersion, Acrycoat L30D (methacrylic acid ethylacrylate), Acrycoat S100 (poly [ethyl acrylate methyl methacrylate]). The effect of these polymers of varying solubility and other physicochemical properties, on the release profile of the drug has been studied and reported in this paper.
Acrycoat E30D, which contains 28.7% solids, is one of the first aqueous polymeric dispersions; it was marked initially in Europe and later I the United States for pharmaceutical applications in the name of Eudragit NE30D. It is prepared by emulsion polymerization and consists of neutral copolymers of ethyl acrylate methyl methacrylate esters that are insoluble over the entire physiological pH range. It is thus suitable for the development of pH independent modified-release oral dosage forms, provided that the solubility of the drug is pH independent. Acrycoat L30D, a 30% aqueous dispersion of copolymer of poly (methacrylic acid ethylacrylate) esters. Copolymers of methyl methacrylic acid and ethyl acrylate as ester components with methacrylic acid are used as enteric coatings, because they contain carboxylic groups that are transformed to carboxylate groups in the pH range of 5-7 by salt formation with alkali and amines. In pure water and diluted acids they form water insoluble films resistant to gastric juices. They are popularly applied in formulating preparations which shows pH dependant drug release. Acrycoat S100, a free flowing powder containing 95% w/w solid polymer is sparingly soluble in water but soluble in alcohol and acetone with 3% v/v water. Being copolymers of Methacrylic acid, they are widely used as slow release enteric coating in tablet and capsule manufacturing industry. They are insoluble in gastric fluid but freely soluble in intestinal fluid of pH 7 and above. They are popularly applied in formulating sustained release pH dependant formulations.
Frusemide (Abdurrahman MA. et al 1992) was selected as the model drug for the experiments. It is a potent high ceiling loop diuretic agent commonly indicated for the treatment of edema of hepatic, cardiac and pulmonary systems during acute or chronic renal failure. In low dose it is a drug of choice for the treatment of chronic hypertension. (Gilbert HM. 1975) It shows a prompt onset of action and produces a peak diuresis far greater than that observed with other diuretic agents. This intense diuresis from a conventional tablet provokes major side effects like electrolytic imbalance manifested in the form of tiredness, dehydration and muscular cramps. (The Extra Pharmacopoeia. 2006) The drug is practically insoluble in water and has a biological half life of 2 hr in patients with renal insufficiency. The aim of the experiment is to produce sustained release micropellets of Frusemide, that can be tabletted or capsulated, exhibiting the same diuretic effect as that of a conventional tablet, but eliminating the toxicity, patient discomfort and non compliances.
MATERIALS AND METHODS
Frusemide was received as a gift sample from Aventis Pharma Ltd., Ankleshwar. Sodium alginate (viscosity of 2% aqueous solution at 25°C was 3500cps) was obtained from Loba Chemie, Mumbai. Calcium chloride dihydrate (A.R. Grade, E.Merck, Germany); Acrycoat E30D, aqueous dispersion (solid content-28.7%w/w) Acrycoat L30D (solid content-30 %w/w) (poly [ethyl acrylate methyl methacrylate]), Acrycoat S100 (solid content-95%w/w) (Methacrylic acid copolymer Type B) ;( Corel Pharmaceuticals, Ahmedabad). All other chemicals were purchased from local supplier in A.R. and L.R. Grade as required.
Preparations of micropellets
The drug (30%w/w) was dispersed uniformly in aqueous mucilage of sodium alginate (2%w/v) using mechanical stirrer maintaining the speed at 500-600 rpm. To this dispersion the desired polymer was mixed in suitable proportions and the entire mixture was stirred for 30 min. The pellets were formed by dropping the bubble free dispersions through a glass syringe into a gently agitated calcium chloride (5% w/v) solution 100 ml. The gelled pellets were cured for 30 min before being filtered and washed thoroughly with distilled water. They are then oven dried for 6 hr at 60°C. The #22 I.P. standard sieve size fractions were used for further studies.
Process variables and Process optimization
The following process variables were investigated (concentration of sodium alginate; concentration of calcium chloride; curing time; height of dropping; variation of drug loading; stirring speed and stirring time) and the different batches thus produced were analyzed for size, shape, ease of preparation, drug content and drug release. On the basis of the result obtained the process parameters were optimized as follows:-
Sodium alginate concentration – 2%w/v
Calcium chloride concentration – 5%w/v
Drug load – 30%w/w
Curing time – 30 min
Height of dropping – 2 cm from the level of CaCl2 solution
Stirring time and speed – 30 min & 500 rpm
Drying condition – oven drying for 6hr at 60°C
Different batches of micropellets were then prepared by using the optimized process variables and the only variation followed was use of different polymers. Nine set of formulations were prepared using Acrycoat E30D (E1, E2, E4); Acrycoat L30D (L1, L2, L4) and Acrycoat S100 (S1, S2, S4) at concentration (1%, 2%, 4%w/w). The final formulations were subjected to several characterization studies.
Characterization of micropellets
Particle size determination
Particle size analysis (Indian Pharmacopoeia. 1996) of the micropellets was done by sieving method using Indian Standard Sieves # 16, #22 and #30. Average particle size was calculated using the formula: - davg = ∑ dn / ∑ n, where n=frequency weight, d= mean diameter. (Table-1)
Scanning electron microscopy
Morphological characterization of the micropellets was done by taking scanning electron micrograph in (JEOL JSM Model 5200, Japan). Cross sectional view were obtained by cutting the micropellets with a razor blade. The samples were coated to 200A° thickness with gold-palladium using (Pelco model 3 sputter coater) prior to microscopy. A working distance of 20nm, a tilt of 0° and accelerating voltage of 15kv were the operating parameters. Micropellets before dissolution were only subjected to SEM study since, after dissolution the pellets become swollen palpable mass. Photographs were taken within a range of 50 - 500 magnifications. (Fig 1-5)
Rheological study (Indian Pharmacopoeia. 1996)
To determine the rheological properties of the micropellets, the angle of repose of all the samples were measured using funnel method. Bulk density was determined by taking known quantity of micropellets in 100ml measuring cylinder and tapping it 3 times from a height of 1 inch at 2 seconds interval. The bulk density was calculated by dividing sample weight by final bulk volume. (Table-1)
Determination of Moisture content (Indian Pharmacopoeia. 1996)
The formulations were subjected to moisture content study, by placing the micropellets at 60° C for 10 minutes in an IR moisture balance. (Table-1)
Loose surface crystal study (LSC) (Abu IK. et al 1996)
This study was conducted to estimate the amount of drug present on the surface of the micropellets which may show immediate release in the dissolution media. 100mg of micropellets (# 22 sizes) were suspended in 100ml of phosphate buffer (pH 6.8), simulating the dissolution media. The samples were shaken vigorously for 15 min in a mechanical shaker. The amount of drug leached out from the surface was analyzed spectrophotometrically at 277.5nm. Percentage of drug released with respect to entrapped drug in the sample was recorded. (Table-1)
Determination of Drug entrapment efficiency
About 100mg of micropellets (# 22 sizes) were accurately weighed and dissolved in 25ml of Phosphate buffer (pH 7.4) for overnight and an aliquot from the filtrate was analyzed spectrophotometrically, after suitable dilution, using SHIMADZU UV-VIS, at 277.5nm. Reliability of the method was judged by conducting recovery analysis using known amount of drug with or without polymer. Recovery averaged 100±0.89%. Drug content of every batch was determined for every size range of micropellets and the mean± S.D.was calculated. Drug Entrapment Efficiency (DEE) was calculated according to the formula % DEE= (Actual drug content/ Theoretical drug content) x 100. (Table-2)
Disintegration studies (Bodmeier R. et al 1989)
Disintegration studies were performed in 0.1N HCl and simulated intestinal fluid (USP XXI) in a rotating bottle apparatus. 5 pellets per vial were kept in 50 ml medium at 37° C and the vials were rotated at 25 rpm. The measured disintegration time was the time taken by the pellets to disintegrate into crystals, the polysaccharide being soluble and the drug insoluble in the disintegrating fluid. (Table-2)
In vitro dissolution study
The USP rotating – paddle Dissolution Rate apparatus (Veego, Mumbai) was used to study drug release from the micropellets. The dissolution parameters [ 100mg pellets ; 37± 2°C ; 50 rpm ; 500ml of USP Phosphate buffer (pH 6.8); n=3; coefficient of variation< 0.05] were maintained for all the nine formulations. 2ml of aliquot were withdrawn at specified intervals and after suitable dilution assayed by SHIMADZU UV-VIS PharmSpec 1700 spectrophotometer at 277.5nm. The data for percent drug release was fitted for zero order and Higuchi matrix equation. The polysaccharide did not interfere with the assay as confirmed from conducting a dissolution study of blank alginate beads. (Table-2)
Determination of stability of the micropellets
The formulations showing the best performance, with respect to in vitro release, from each set of formulations were stored at 4°C, room temperature and 45°C for a month. Every week samples were withdrawn and were assayed spectrophotometrically at 277.5 nm using Phosphate buffer (pH 6.8) as blank. (Table- 3)
RESULTS AND DISCUSSION
The micropellets were prepared in an environment free from organic solvents by dropping a mixture of colloidal copolymer dispersion, the dispersed drug Frusemide, and mucilage of sodium alginate in calcium chloride solution, which acted as a counterion. The droplets instantaneously formed gelled spherical beads due to cross linking of calcium ion with the sodium ion which remained ionized in the solution. Smaller particle can be prepared by adjusting the height of the syringe from the level of counterion solution, compression force on the plunger of the syringe. The gelled particles were cured to get sufficiently hardened and then filtered and dried. The colloidal polymer particles fused into the polymer matrix during drying with the drug being dispersed in the latex. The micropellets thus formed using three different polymers did show significant results on evaluation.
The size of the micropellets ranged between 540 µm to 800 µm and increased significantly with the concentration of the copolymers. The average particle size was on the highest side with Acrycoat E30D polymers followed by S100. The particle size distribution was uniform and narrow. It can be estimated that with further increment in the copolymer concentration the particles would change from micro to granular level.
The scanning electron micrograph (Figure 6-8) shows the pellets being discoid in shape. Surface depression was noticed at the point of contact on the drying paper. On comparison of the pellets prepared from three polymers in highest concentration, it was evident from the photograph that more roughness with Acrycoat E30D copolymers was achieved than that of the other two. Acrycoat L30D giving the most smooth surfaced particle. It can be concluded that the roughness is due to the density of the matrix which in turn justifies its sustained release. The dense network of drug-polymer-copolymer increases the tortuisity, as evident from Figure-9, thus delaying the release of the drug and retarding the penetration of water required to make the pellets swell for disintegration. The micrograph of the blank pellets (Figure-5) act as a control and suggests that increase in total weight of the pellets makes it more spherical.
The rheological parameters like angle of repose and bulk density of all the pellets (Table-1) confirms better flow and packing properties. Thus, the micropellets if tabletted or encapsulated, requires less amount of lubricants and ensures low production cost leading to its feasibility for large scale production.
Loose surface crystal (LSC) study was an important parameter giving an indication of the amount of drug on the surface of the micropellets without proper entrapment. With the increase in the copolymer concentration % LSC decreased significantly owing to high entrapment of drug in the dense network of polymers.
Low moisture content in all the micropellets indicates the effectiveness of the optimized drying condition. Low moisture level ensures better stability of the drug in the micropellets.
Significantly high entrapment efficiency of drug with Acrycoat L30D (Table-2) over other polymers confirms it being more rigid among the three.
As described during the discussion on the photomicrograph, formulation E4 showed highest disintegration time which may be due to its stronger latex network structure. The micropellets being less porous among the three, delays the penetration of water needed for swelling and eventual disintegration. No disintegration was observed in 0.1N HCl, even when the samples were kept for overnight in the medium confirming with the fact that all the polymers investigated are insoluble in gastric pH and over and above pH 5.5. The ionic character of the polysaccharide alginate also resulted in pH dependant disintegration of the micropellets. Acrycoat E30D consists of neutral copolymers of ethyl acrylate methyl methacrylate esters that are insoluble over the entire physiological pH range. It is thus suitable for the development of pH independent modified-release oral dosage forms, provided that the solubility of the drug is pH independent. The other two acrylic polymers show pH dependent release.
The in vitro release data of all the formulations were fitted in Zero order and Higuchi matrix model and the rate constants and correlation coefficient were compared to get a trend in the release pattern of the drug from the formulations. From Table -2, comparing the R2 value of both the kinetic models, it is evident that all the batches predominantly showed zero-order release. The formulations of E30D sustained the release of the drug for more than 8 hours while for the formulations of L30D and S100, the release varied depending on their concentrations, within a range of 5-8 hours (Figure 1-3) as compared with the conventional tablet dosage form (Figure-4). Predominantly, the drug gets released by passive diffusion through water filled pores. The loose drug on the surface of the micropellets, on release, exposes the pores or micro channels, through which diffusion of the drug present in the inner matrix occurs. Due to loose drug present on the surface of the micropellets (LSC) the in vitro release profile obtained indicated a biphasic pattern i.e. initial fast release followed by a sustained pattern. Batches of Acrycoat E30D micropellets showed more prolonged action as evident from its t50 values when compared with other two acrylic polymers. Increase in the polymer concentration increased the crosslink density thereby creating barrier for drug diffusion, hence more prolongation.
When studied for stability at 4°C, room temperature and 45°C for a month, the drug was found to be stable at 4°C and room temperature and all the formulations showed gradual degradation at high temperatures.
CONCLUSION
Sustained release micropellets containing water insoluble drug were successfully prepared employing ionotropic gelation technique, entirely avoiding the use of organic solvents. Apart from the natural water soluble polymer, namely, sodium alginate, the use of copolymer further prolongs the release of the drug. Acrylic based colloidal polymer dispersions (Acrycoat E30D) showed good encapsulation efficiencies and maximum prolongation of drug release. Hence, further studies can be extended taking Acrycoat E30D as the release controlling copolymer. Considering the end product, the micropellets could be administered as prepared or could be compressed into tablet or filled in capsule shell. The entire process is feasible in an industrial scale and demands pilot study.
ACKNOWLEDGEMENTS
Authors wish to thank Aventis Pharmaceuticals (Ankleshwar, India) for providing gift sample of Frusemide and Corel Pharmaceuticals (Ahmedabad, India) for providing gift sample of Acrycoat E30D, Acrycoat L30D, and Acrycoat S100. We are also thankful to the staff of University Science Instrumentation centre (USIC), Jadavpur University, Kolkata for their relentless cooperation in SEM study.
TABLE- 1
COMPARATIVE STUDY OF VARIOUS PHYSICAL PARAMETERS FOR ALGINATE MICROPELLETS CONTAINING FRUSEMIDE AND RELEASE RETARDED WITH ACRYCOAT E30D, L30D AND S100 RESPECTIVELY
Formulation Code |
Acrycoat Composition (% w/w) |
Moisture content (% ± S.D.) |
LSC with respect to Entrapped Drug (%) |
Mean Diameter (µm ± S.D.) |
Angle of repose θ ± S.D.) |
Bulk density (g/cc ±S.D.) |
E1 |
E30D-1% | 1.48 ± 0.48 |
3.549 |
608.16 ± 0.59 |
18.32 ± 0.79 |
0.658 ± 0.68 |
E2 |
E30D-2% | 1.44 ± 0.56 |
2.369 |
760.89 ± 0.51 |
20.56 ± 1.03 |
0.674 ± 1.52 |
E4 |
E30D-4% | 2.23 ± 0.68 |
1.567 |
782.78 ± 0.36 |
21.24 ± 1.97 |
0.682 ± 1.96 |
L1 |
L30D-1% | 2.83 ± 0.67 |
4.318 |
547.29 ± 0.54 |
17.53 ± 1.12 |
0.617 ± 0.84 |
L2 |
L30D-2% | 2.14 ± 0.46 |
3.878 |
613.58 ± 0.72 |
20.78 ± 1.48 |
0.646 ± 1.48 |
L4 |
L30D-4% | 1.67 ± 0.86 |
2.972 |
704.26 ± 0.22 |
22.19 ± 2.07 |
0.677 ± 2.36 |
S1 |
S100-1% | 2.21 ± 0.41 |
3.943 |
594.57 ± 0.43 |
18.24 ± 1.31 |
0.651 ± 1.19 |
S2 |
S100-2% | 1.91 ± 0.26 |
3.018 |
678.32 ± 0.56 |
21.33 ± 1.78 |
0.673 ± 1.76 |
S4 |
S100-4% | 1.76 ± 0.61 |
2.454 |
767.25 ± 0.31 |
22.53 ± 2.19 |
0.680 ± 2.48 |
* Results shown are the mean ± SD. n = 6 for mean diameter and n = 3 for moisture content, angle of repose, bulk density and LSC study.
TABLE- 2
COMPARATIVE STUDY OF VARIOUS PHARMACEUTICAL FACTORS FOR ALGINATE MICROPELLETS CONTAINING FRUSEMIDE AND RELEASE RETARDED WITH ACRYCOAT E30D, L30D AND S100 RESPECTIVELY
Formulation Code |
Acrycoat Composition (% w/w) |
Drug Entrapment Efficiency (% ± S.D.) |
Disintegration Time (min) |
t50 ( min ) |
Zero order Higuchi SQRT K0 R2 KH R2 |
|||
E1 |
E30D-1% | 94.48 ± 0.48 |
42 |
272 |
9.3247 |
0.9477 |
25.479 |
0.7939 |
E2 |
E30D-2% | 93.44 ± 0.56 |
64 |
290 |
9.0388 |
0.9463 |
23.909 |
0.7857 |
E4 |
E30D-4% | 91.23 ± 0.68 |
97 |
341 |
7.9428 |
0.8407 |
20.151 |
0.6492 |
L1 |
L30D-1% | 99.22 ± 0.43 |
31 |
92 |
18.622 |
0.9519 |
44.784 |
0.9558 |
L2 |
L30D-2% | 98.56 ± 0.43 |
43 |
153 |
14.435 |
0.9776 |
35.857 |
0.8773 |
L4 |
L30D-4% | 97.68 ± 0.43 |
64 |
267 |
10.727 |
0.9123 |
27.225 |
0.7444 |
S1 |
S100-1% | 91.22 ± 0.34 |
38 |
119 |
17.496 |
0.9844 |
38.485 |
0.9329 |
S2 |
S100- 2% | 86.09 ± 0.85 |
51 |
181 |
13.333 |
0.9438 |
30.280 |
0.8428 |
S4 |
S100-4% | 83.58 ± 0.91 |
79 |
278 |
10.061 |
0.9467 |
23.862 |
0.7944 |
* K0 , KH – Release Rate Constants for Zero Order and Higuchi release Kinetic Model respectively
* R2 – Correlation coefficient.
* Results shown are the mean ± SD. n = 3 for disintegration study, entrapment efficiency and dissolution study.
TABLE- 3
ACCELERATED STABILITY STUDIES OF FRUSEMIDE MICROPELLETS PREPARED WITH DIFFERENT POLYMERS
TIME |
S4 |
L4 |
E4 |
||||||
(WEEK) |
4°C |
RT |
45°C |
4°C |
RT |
45°C |
4°C |
RT |
45°C |
0 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
1 |
98.36 |
98.23 |
87.39 |
99.16 |
97.98 |
87.47 |
99.28 |
98.52 |
89.28 |
2 |
97.38 |
96.29 |
82.54 |
96.79 |
96.61 |
85.18 |
97.57 |
96.93 |
88.42 |
3 |
94.85 |
94.36 |
77.33 |
94.82 |
94.21 |
83.84 |
96.62 |
95.89 |
86.74 |
4 |
90.52 |
91.72 |
75.94 |
91.39 |
92.22 |
80.71 |
95.09 |
95.37 |
85.33 |
RT--- ROOM TEMPERATURE
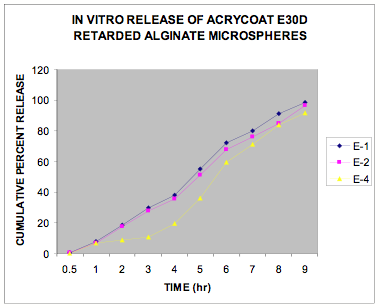
Figure 1
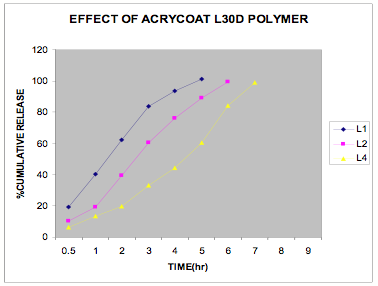
Figure 2
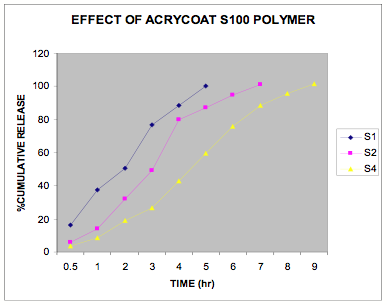
Figure 3
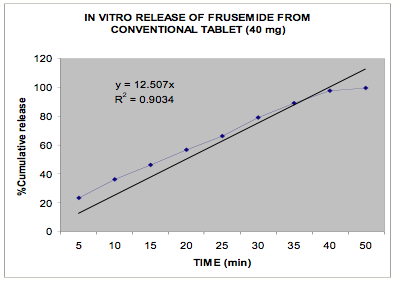
Figure 4
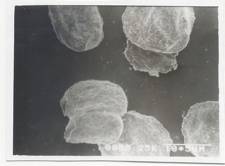
Figure 1- SEM Photograph of blank alginate micropellets (50X)
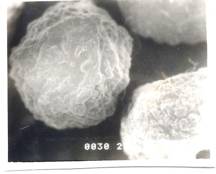
Figure 6- SEM Photograph of Frusemide loaded alginate micropellets with Acrycoat E30D (4%w/w) (50X) Formulation # E4
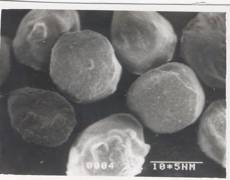
Figure 7 - SEM Photograph of Frusemide loaded alginate micropellets with Acrycoat L30D (4%w/w) (50X) Formulation # L4
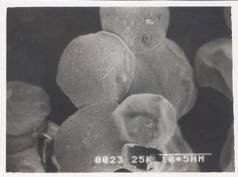
Figure 8 - SEM Photograph of Frusemide loaded alginate micropellets with Acrycoat S100 (4%w/w) (50X) Formulation # S4
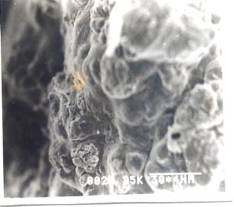
Figure 9- SEM Photograph of Frusemide loaded alginate micropellets with Acrycoat E30D (4%w/w) (350X) Formulation # E4
REFERENCES
Abdurrahman MA, Fahad JA. (1992) Frusemide. Analytical Profiles of Drug Substances and Excipients. Aca Press Inc. ed Florey K. 18: 153-193
Abu IK, Gracia CL, Robert LD. (1996) Preparation and evaluation of zidovudine-loaded sustained-release microspheres. J Pharm Sci. 85(6): 575-576
Baken JA. (1987) Microencapsulation.In: Lachman and Lieberman (Eds) The Theory and Practice of Industrial Pharmacy. 3rd ed. Varghese Publishing House, Mumbai. 412-429
Bodmeier R, Chen H. (1991) Pseudoephedrine HCl microspheres formulated into an oral suspension dosage form.J.Controlled release.15: 65-77
Bodmeier R, Paeratakul O. (1989) Spherical Agglomerates of water insoluble drugs. J.Pharm. Sci. 78: 964-967.
Bodmeier R, Wang J. (1993) Microencapsulation of Drugs with Aqueous Colloidal dispersion. J.Pharm.Sci. 82(2):191-194
Gilbert HM. (1975) The Pharmacological basis of Therapeutics. 6th ed. New York: McMillan Co. Inc. 903
Indian Pharmacopoeia. (1996) 5th ed. Controller of Publication. 328-329,393-395
JamesW.McGinity, (1997) Aqueous polymeric coatings for pharmaceutical dosage forms, 2nd ed. 355,381
Lehmann K. (1989) In. Aqueous Polymeric Coatings for Pharmaceutical Applications. McGinity JW. eds. New York: Marcel Dekker. 153-245
Lim F, Sunn AM.(1980) Microencapsulated islets as bioartificial endocrine pancreas. Science. 210: 908-910.
Lim LY, Wan SC. (1997) Propranolol hydrochloride binding in calcium alginate bead. Drug Dev Ind Pharm. 23 (10): 973-980.
Segi N, Yotsuyanagi T. Ikeda K. (1989) Interaction of Calcium- induced Alginate Gel beads with Propranolol. Chem Pharm Bull, 37 (11), 3092-3095.
Steuernagel CR. (1989) In. Aqueous Polymeric Coatings for Pharmaceutical Applications.McGinity JW. eds. New York: Marcel Dekker. 1-61
The Extra Pharmacopoeia, “ Martindale”, The Pharmaceutical Press, 34th edition,2006; 919-922
First Published April 2007
Copyright © Priory Lodge Education Limited 2007
All pages copyright ©Priory Lodge Education Ltd 1994-

