| |
| Introduction | Structure | Receptor Affinities | Pharmacokinetics |
| Clinical Efficacy | Tolerability | Conclusions | Review article by Meats |
Keywords
Schizophrenia - quetiapine - antipsychotic - neuroleptic - atypical - pharmacology
Summary
Quetiapine fumarate, Bis [2-(2-[4-(dibenzo[b,f][1,4]thiazepin-11-yl]ethoxy)ethanol] fumarate (IUPAC)2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]-ethanol-(E)-2-butanedioate (2:1) salt), [ICI 204,636], is a novel dibenzothiazepine antipsychotic developed by Zeneca. It is marketed under the trade name ‘Seroquel’Seroquel. Quetiapine is well tolerated and clinically effective in the treatment of schizophrenia.
The initial hope of investigators was that quetiapine would have antipsychotic potential and that it might share some of the properties of clozapine without its toxicity to white blood cells.
The effective dosage range is usually 300-450 mg/day split into two doses. The dose is titrated upwards from 25 mg twice dailybd from day one to 300mg/daya fuller dosage on day 4. Elderly patients or patients with liver problems should be started on lower doses. It is both superior to placebo and, and comparable to haloperidol in reducing positive symptoms at doses ranging from 150 to 750 mg/day and is an effective treatment for in reducing negative symptoms at a dose of 300 mg/day.
Somnolence is the most common adverse event. Abnormalities of the QTqt interval on ECG appear very infrequently and there is no need for a baseline ECG or blood pressure monitoring as used to be the case with ssertindole. There is no need for haematological monitoring as with clozapine. Quetiapine, across the full dosage range, is associated with no greater extrapyramidal symptoms than placeboThere is a reduced potential for extrapyramidal symptoms compared with conventional antipsychotics.
Quetiapine’s general efficacy and side effect profile suggest that, unless there are unforeseen post-marketing complications, quetiapine deserves a major place in the initial and long term management of schizophreniform disorders.
Figure One: The molecular structure of quetiapine
Quetiapine fumarate, Bis [2-(2-[4-(dibenzo[b,f][1,4]thiazepin-11-yl]ethoxy)ethanol] fumarate (IUPAC) 2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]-ethanol-(E)-2-butanedioate (2:1) salt), [ICI 204,636], is a novel dibenzothiazepine antipsychotic developed by Zeneca Pharmaceuticals. It is marketed under the trade name ‘Seroquel’. Quetiapine is well tolerated and clinically effective in the treatment of schizophrenia.
Clinical Indications
Licensed indications vary from country to country. The following indications are derived from MEDLINE, Cochrane and PSYCLIT database searches. The primary indications are adult schizophrenia and related psychoses in adults and the elderly (Arvanitis & Miller, 1997, McManus etal, 1999). Affective and aggressive symptoms in schizophrenia (Hellewell et al, 1998), adolescent schizophrenia, schizoaffective disorder, bipolar affective disorder and mania, affective psychoses, behaviour and symptom control in dementia could form secondary indications, but more studies are awaited. The relatively greater ability to reduce negative symptoms compared to conventional antipsychotics provides an additional indication.
Indications that have cropped up in the literature include antipsychotic therapy in patients with Parkinson's - where there was a low propensity to aggravate motor symptoms (Parsa & Bastani, 1998).
Quetiapine and other antipsychotics have been proposed as first-line treatments for schizophrenia and there is some rationale for this (Lieberman, 1996). Clozapine has not been generally used as a first-line treatment due to its potential for agranulocytosis. Compared to conventional antipsychotics such as chlorpromazine and haloperidol, quetiapine and other atypical antipsychotics provide superior efficacy or, fewer less side effects, particularly extrapyramidal symptoms (EPS), and the possibility of better patient compliance. Drugs. Therefore the best use of drugs such as quetiapine may therefore be particularly appropriate in patients at the beginning of their illness as a first-line treatment of schizophrenia.
Health economic studies are currently ongoing.
Pre-clinical Studies
In rodents, quetiapine produced mild levels of catalepsy, which suggested antipsychotic potential. It has a low propensity to cause dystonia in drug-na´ve or haloperidol sensitised monkeys (Hirsch et al, 1996). Rats treated with apomorphine (APO) exhibit deficits in prepulse inhibition (PPI) of the acoustic startle response. This is thought to model schizophrenia. Clozapine restores PPI in APO-treated rats and this ability tends to correlate (Rs = 0.991) with the clinical potency of antipsychotics. Quetiapine similarly restores deficits in PPI, (Swerdlow et al, 1994).
In vitro binding affinity is more for 5-HT2 receptors than dopamine DA2 receptors and in vivo dopamine DA2 and 5-HT2 receptor occupancies are 27% and 58% respectively (see Table One).
Receptor affinities in the brain |
||
Receptor |
Quetiapine(IC50, nM) |
Clozapine(IC50, nM) |
DA2 |
329 |
132 |
DA1 |
1268 |
322 |
5-HT2 |
148 |
20 |
5-HT |
717 |
316 |
alpha1 |
94 |
50 |
alpha2 |
271 |
28 |
H1 |
30 |
23 |
Sigma |
90 |
>10,000 |
Muscarinic |
>10,000 |
287 |
Benzodiazepine |
>5000 |
>5000 |
Table One: Receptor affinities in the brain
Conventional antipsychotics block up to 80% of DA2 receptors as measured using Positron Emission Tomography scans - see Figure Two (Kasper et al, 1997). Clozapine and quetiapine occupy about 30%, which may explain their low propensity to cause EPS. Only clozapine and quetiapine share a low affinity for dopamine DA2 receptors and an antagonistic action at alpha2 adrenoceptors (Reynolds et al, 1998). The clinical import of this is not yet clear.
Compared to clozapine, quetiapine has much the same ratio of DA2/5HT2 occupancy (Gefvert et al, 1998).
Quetiapine binds to dopamine DA2 receptors in the striatum and occupies 44% of receptors 2 hours (t[max]) after the last dose. After 26 hours occupancy dropped to the same level as in untreated healthy volunteers. Serotonin 5HT2 receptor blockade in the frontal cortex was 72% after 2 hours, which fell to 50% after 26 hours. The terminal plasma half-life of quetiapine was found to be 5.3 hours (Gefvert et al, 1998).
Pharmacology and pharmacokinetics
Quetiapine is rapidly absorbed and has linear pharmacokinetics. The mean half-life for 375 mg/day is 6.9 hours. The steady state pharmacokinetics appear to be the same for men and women. At 2, 12 and 24 hours post dose quetiapine's 5-HT2 occupancy was 72%, 48% and 50% respectively. It is only moderately bound to plasma protein (83%). Quetiapine is extensively metabolised by the liver involving mainly sulphoxidation by cytochrome P450 3A4 (Gunasekara & Spencer, 1998). It is extensively metabolised to over 20 metabolites - some are active, but the concentration of these active metabolites is low compared with the parent compound. Quetiapine is eliminated primarily in the form of metabolites, only 5% being excreted unchanged. About 73% is excreted in the urine and 21% in the faeces. Its half-life is about six hours.
Oral clearance in the elderly is half that of younger patients. Clearance is also reduced by hepatic or renal impairment.
Quetiapine binds to dopamine DA2D2 receptors in the striatum and occupies 44% of receptors 2 hours (t[max]) after the last dose. After 26 hours occupancy dropped to the same level as in untreated healthy volunteers. Serotonin 5HT2 receptor blockade in the frontal cortex was 72% after 2 hours, which fell to 50% after 26 hours. The terminal plasma half-life of quetiapine was found to be 5.3 hours (Gefvert et al, 1998).
Conventional antipsychotics block up to 80% of DA2 receptors as measured using PET scans (Kasper et al, 1997). Clozapine and quetiapine occupy about 30%, which may explain their low propensity to cause EPS.
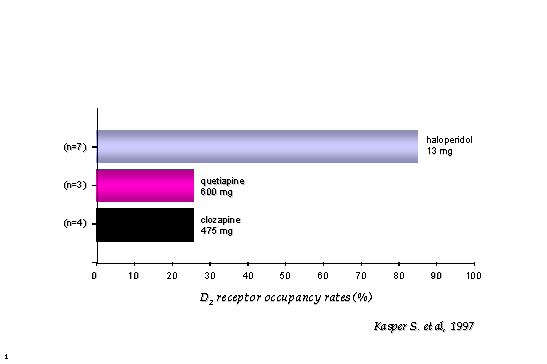
Figure 2: Positron Emission Tomography (PET) derived DA2 occupancy comparing quetiapine, clozapine and haloperidol.
Pharmacokinetics and Metabolism
Quetiapine is rapidly absorbed and has linear pharmacokinetics. The mean half-life for 375 mg/day is 6.9 hours. The steady state pharmacokinetics appear to be the same for men and women. At 2, 12 and 24 hours post dose quetiapine's 5-HT2 occupancy was 72%, 48% and 50% respectively. It is only moderately bound to plasma protein (83%). Quetiapine is extensively metabolised by the liver involving mainly sulphoxidation by cytochrome P450 3A4 (Gunasekara & Spencer, 1998). It is extensively metabolised to over 20 metabolites - some are active, but the concentration of these active metabolites is low compared with the parent compound. Quetiapine is eliminated primarily in the form of metabolites, only 5% being excreted unchanged. About 73% is excreted in the urine and 21% in the faeces.
Oral clearance in the elderly is half that of younger patients. Clearance is also reduced by hepatic or renal impairment.
Only clozapine and quetiapine share a low affinity for dopamine DA2 receptors and an antagonistic action at alpha2 adrenoceptors (Reynolds et al, 1998). The clinical import of this is not yet clear.
Compared to clozapine, quetiapine has much the same ratio of DA2/5HT2 occupancy (Gefvert et al, 1998).
Pre-clinical Studies
The initial hope of investigators was that quetiapine would have antipsychotic potential and that it might share some of the properties of clozapine without its toxicity to white blood cells.
In rodents quetiapine produced mild levels of catalepsy, which suggested antipsychotic potential. It has a low propensity to cause dystonia in drug-na´ve or haloperidol sensitised monkeys (Hirsch et al, 1996). Rats treated with apomophrine (APO) exhibit deficits in prepulse inhibition (PPI) of the acoustic startle response. This is thought to model schizophrenia. Clozapine restores PPI in APO-treated rats and this ability tends to correlate (Rs = 0.991) with the clinical potency of antipsychotics. Quetiapine similarly restores deficits in PPI, (Swardlow et al, 1994).
In vitro binding affinity is more for 5-HT2 receptors than dopamine D2 receptors and in vivo dopamine D2 and 5-HT2 receptor occupancies are 27% and 58% respectively. Quetiapine is totally devoid of D4 activity, (Schotte et al, 1996).
In one of Zeneca’s initial clinical trials, five fixed doses of quetiapine were studied to investigate the dose-response relationship (Arvanitis & Miller, 1997). Outcome measures included the Brief Psychiatric Rating Scale (BPRS), Clinical Global Impression (CGI), and the Modified Scale for the Assessment of Negative Symptoms (SANS). Three hundred sixty-one patients with acute exacerbations of chronic schizophrenia (DSM-III-R) from 26 US centres entered this double blind, placebo-controlled trial with acute exacerbations of chronic schizophrenia (DSM-III-R). After a single blind, placebo washout phase patients were randomised to double blind treatment with quetiapine (75, 150, 300, 600, or 750 mg daily), haloperidol (12 mg daily), or placebo and evaluated weekly for 6 weeks. At the end of the study, significant differences (p < 0.05, analysis of covariance) were identified between the four highest doses of quetiapine and placebo for BPRS total, BPRS positive-symptom cluster, and CGI Severity of Illness item scores and between quetiapine 300 mg and placebo for SANS summary score. The study’s scales did not show a statistically significant difference between quetiapine and haloperidol. No significant safety problems manifested as the dose increased. Quetiapine at any of the doses seemed no different from placebo in terms of the incidence of extrapyramidal symptoms
Similar efficacy studies demonstrate that quetiapine is as effective as or superior to chlorpromazine (Peuskens & Link, 1997,; also Small et al,1 Borison and Arvanitis997, Borison et al 1996, Arvanitis & Miller, 1997). A 6-week, double blind, randomized, multicentre, parallel-group study compared the efficacy of quetiapine (n=101) with that of chlorpromazine (n=100) in hospital patients with acute exacerbation schizophrenia or schizophreniform disorder. The mean daily doses of quetiapine and chlorpromazine at the end of the study were 407 mg and 384 mg, respectively. Both treatments reduced positive and negative symptoms, with a trend towards superior efficacy for quetiapine. The quetiapine group had a lower incidence of adverse events and a lower incidence of treatment-emergent extrapyramidal symptoms requiring treatment with anti-cholinergics. Quetiapine was not associated with an increase in serum prolactin supporting the preclinical profile of quetiapine as an atypical antipsychotic agent.
There are now over several short-term (6 weeks or so) double blind randomised clinical trials on quetiapine's efficacy, producing data from over 1,000 patients (referred to above). Quetiapine showed consistent efficacy in the treatment of positive symptoms using the Brief Psychiatric Rating Scale (BPRS) positive symptom cluster - comparable to other antipsychotic agents., such as haloperidol and chlorpromazine Longer-term efficacy studies are being performed. Quetiapine may appear to improve mood in patients with schizophrenia according to ratings on the BPRS item scores for depressive mood, anxiety, guilt and tension. Cognitively there is some evidence (Sax et al, 1998) that attentional performance in patients with schizophrenia improves with quetiapine, and after 2 months, does not differ significantly from that of the controls.
Leucht et al's meta-analysis study (1999) of risperidone, olanzapine, sertindole and quetiapine suggest a similar efficacy profile compared to placebo, but that olanzapine, sertindole and quetiapine had more favourable EPSE profiles.
Adverse Event |
Percentage of patients in Phase II/III studies (n=1710) |
Somnolence |
18.2 |
Dizziness |
7.5 |
Asthenia |
4.3 |
Postural hypotension |
5.8 |
Tachycardia |
4.2 |
Constipation |
5.5 |
Dry mouth |
7.1 |
Dyspepsia |
3.4 |
Sexual dysfunction |
0.5 |
Table Two: Incidence of Side Effects with Quetiapine
Interestingly placebo controlled studies of quetiapine identified no significant difference between quetiapine and placebo for dystonia, akathisia and Parkinsonism (Small et al, 1997). There appear to be no dose-related increases in the incidence of EPS with quetiapine, unlike some other antipsychotics e.g. risperidone. Only 8.6% of patients in clinical trials required anticholinergic medication compared to 12.6% of patients on placebo. There were significantly lower incidences of EPS in comparisons of quetiapine and haloperidol.
Excessive sedation has been reporte as a consequnce of overdose (Harmon et al, 1998)
Patient satisfaction is rarely measured, but is probably an important factor in compliance. Seventy six per cent of 129 patients on quetiapine for at least six months in an open label study extension reported that they were very or extremely satisfied with their treatment in a questionnaire based study for Zeneca (Langham & McKellar, 1998). 74% of these long-term patients reported no side effects and 23% only mild side effects, in the previous month of therapy. Ninety-seven per cent said they preferred quetiapine to previous medications.
Quetiapine is generally well tolerated by the elderly, although lower doses need to be given because of its pharmacokinetics. There are negligible EPS in the elderly. Somnolence, dizziness and postural hypotension are the most frequent side effects in the elderly, (Yeung et al, 1998).
Reproductive and hormonal adverse events (e.g. gynaecomastia, amenorrhoea, abnormal ejaculation, lactation) occur very rarely, probably since prolactin levels are no different to placebo across the dose range.t affected (Goldstein, 1998).
Drug interactions
Phenytoin is a CYP3A4 inducer that may lead to an increase in the oral clearance of quetiapine if co-administered. Other hepatic inducers may produce similar interactions (e.g. carbamazepine, phenytoin, rifampicin, and barbiturates).
No interactions are seen with fluoxetine, lithium, imipramine, haloperidol, risperidone and lorazepam (single dose).
Quetiapine is an effective treatment for both acts against so-called positive and negative symptoms of schizophrenia, with similar overall efficacy to haloperidol and chlorpromazine
Quetiapine may improve depressed mood in schizophrenia
Quetiapine has a lower incidence of EPS than haloperidol, one of psychiatrists' favourite first line treatments for schizophrenia
No difference between placebo and quetiapine regarding EPS across the dose range
Quetiapine does not raise prolactin levels or produce frequent reproductive or sexual side effects
No ECG, blood pressure or haematological monitoring required
Few drug interactions
Quetiapine has a very well-tolerated side effect profile and in long term open label extension studies, is associated with high levels of patient acceptability and satisfactionis popular with patients
Quetiapine is an atypical antipsychotic that was launched in the UK in 1997. The clinical impression post-marketing is of a useful antipsychotic with few EPSEs and with a potential to reduce positive and negative symptoms to a better degree than some conventional antipsychotics and some other ‘atypical’ antipsychotics. There is good tolerability in humans. Somnolence is the most common adverse event. Quetiapine’s general efficacy and side effect profile suggest that, other than any unforeseen post-marketing complications, quetiapine deserves a major place in the first line and long term management of schizophrenic psychotic disorders.
Correspondence: Dr Ben Green, MRCPsych, Senior Lecturer in Psychiatry, University Department of Psychiatry, Royal Liverpool University Hospital, The University of Liverpool, UK.
Email: bengreen@liverpool.ac.uk
References and further reading
Aizenberg, D, Zemishlany, Z, Dorfman-Etrog P, Weizman, A. (1995). Sexual dysfunction in male schizophrenic patients. J Clin Psychiatry, 56(4): 137-141.
Arvanitis, L.A., Miller, B.G. (1997) TI: Multiple fixed doses of "Seroquel" (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The ‘Seroquel’Seroquel Trial 13 Study Group.Biol. Psychiatry. 42(4): 233-46
Borison RL, Arvanitis LA, Miller BG :(1996) ICI 204,636, an atypical antipsychotic: Efficacy and safety in a multicenter, placebo-controlled trial in patients with schizophrenia. J Clin Psychopharmacol 16/2: 158-169 .
Casey, D.E., (1996) Side effect profiles of new antipsychotic agents. J.Clin.Psychiatry. 57 Suppl 11: 40-5; discussion 46-52
Currier, G.W., Simpson, G.M. (1998) Antipsychotic medications and fertility. Psychiatric-Services. Vol 49(2): 175-176
Ereshefsky, L. (1996) Pharmacokinetics and drug interactions: update for new antipsychotics.J.Clin.Psychiatry. 57 Suppl 11: 12-25
Fabre LF, Arvanitis L, Pultz J & al:(1995) ICI 204,636, novel, atypical antipsychotic: Early indication of safety and efficacy in patients with chronic and subchronic schizophrenia. Clin Ther 17/3: 366-378.
Fulton B, Goa KL: ICI 204,636. (1995) An initial appraisal of its pharmacological properties and clinical potential in the treatment of schizophrenia. CNS Drugs 4/1: 66-78.
Gefvert, O, Bergstrom, M, Langstrom, B, Lundberg, T, Lindstrom, L, Yates, R. (1998) Time course of central nervous dopamine-D2 and 5-HT2 receptor blockade and plasma drug concentrations after discontinuation of quetiapine (‘Seroquel’Seroquel) in patients with schizophrenia. Psychopharmacology-Berl. 135(2): 119-26
Goldstein, J M. (1998) Low incidence of reproductive/hormonal side effects with s’Seroquel’eroquel (quetiapine) is supported by its lack of elevation of plasma prolactin concentrations. Poster presentation, CINP.
Gunasekara N S & Spencer C M (1998) Quetiapine - a review of its use in schizophrenia. CNS Drugs 9 (4) 325-340.
Harmon TJ; Benitez JG; Krenzelok EP; Cortes Belen E (1998)
Loss
of consciousness from acute quetiapine overdosage. J Toxicol Clin Toxicol, 1998, 36:6, 599-602
Hellewell, J S E, McKellar, J, & Raniwalla, J.
(1998) Seroquel: efficacy in aggression, hostility and low mood of
schizophrenia. Poster presentation, CINP. Hirsch, S R, Link, C G G, Goldstein, J M &
Arvanitis, L A. (1996) ICI 204, 636: a new atypical antipsychotic drug. Brit. J
Psychiatry, 168 (suppl. 29), 45-46. Kasper S (1997) Dopamine D2 receptor binding in typical
and atypical neuroleptics. Biol Psychiatry; 41 (75): 67S Abs 227. Langham, S & McKellar, J. (1998) Patient
satisfaction and acceptability of long-term treatment with ‘Seroquel’.
Zeneca Pharmaceuticals.
Leucht S, Pitschel Walz G, Abraham D,
Kissling W. (1999) Efficacy and extrapyramidal side-effects of the new
antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to
conventional antipsychotics and placebo. A meta-analysis of randomized
controlled trials.
Schizophr Res, , 35:1, 51-68.
Lieberman, J.A. (1996) Atypical antipsychotic drugs as
a first-line treatment of schizophrenia: a rationale and hypothesis. J.Clin.Psychiatry; 57
Suppl. 11: 68-71. McManus DQ, Arvanitis LA, Kowalcyk BB.
(1999) Quetiapine, a novel antipsychotic: experience in elderly patients with
psychotic disorders. Seroquel Trial 48 Study Group. .J Clin Psychiatry,
60:5, 292-8.
Nutt, D J (1994) Putting the ‘A’ in atypical: does alpha-2 adrenoceptor antagonism account for the therapeutic advantage of new antipsychotics? J Psychopharmacol., 8, 193-5.
Parsa MA, Bastani B. (1998)
Quetiapine (Seroquel) in the treatment of psychosis in patients with Parkinson's
disease.
J
Neuropsychiatry Clin Neurosci, 10:2, 216-9.
Peuskens, J. & Link, C.G.(1997) A comparison of
quetiapine and chlorpromazine in the treatment of schizophrenia. Acta-Psychiatr-Scand.
96(4): 265-73. Reynolds, G P, Tillery, C , Elliott, J, & Blake, T
J. (1998) Human alpha2 adrenoceptor subtypes and their role in antypical
antipsychotic drug action. (Poster presentation, CINP). Sax KW, Strakowski SM, Keck PE.
(1998) Attentional improvement following quetiapine fumarate treatment in
schizophrenia. Schizophr Res, 33:3, 151-5 Schotte, A., Janssen, P.F., Gommeren, W., et al. (1996)
: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo
receptor binding. Psychopharmacology-Berl. 124(1-2): 57-73 Small, J G, Hirsch, S R, Arvanitis, L A, Miller, B G,
Link, C G. (1997) Quetiapine in patients with schizophrenia. A high- and low-dose
double-blind comparison with placebo. Arch. Gen. Psychiatry. 54(6): 549-57 Swerdlow, N.R., Zisook,D., Taaid, N. (1994) Seroquel (ICI 204,636) restores prepulse inhibition of acoustic
startle in apomorphine-treated rats: Similarities to clozapine. Psychopharmacology-Berl.
114(4): 675-8 Wong JYW, Ewing BJ, Fabre LF & al:(1996)
Multiple-dose pharmacokinetics of '‘Seroquel’Seroquel ' (ICI 204,636) in
schizophrenicschizuphrenic men and women. Eur Psychiatry 11(suppl 4): 429s-430s. Yeung, P, Hellewell, J S E, Raniwalla J & Atkinson.
(1998) Seroquel: extrapyramidal symptoms and tolerability profile in
the elderly. Poster presentation, CINP.
All pages copyright ęPriory Lodge Education Ltd 1994-2006.