A multi-centre, double-blind comparative study of the efficacy and safety of aceclofenac and diclofenac in the treatment of rheumatoid arthritis
Current Medical Research Opinion (1995), 13, 305
G. Pasero, M.D.1,
R. Marcolongo, M.D.2,
U. Serni, M.D.3,
M.J. Parnham, Ph.D.4,
and
F. Ferrer M.D.5
1 Institute of Medical Pathology,
Department of Rheumatology,
St. Chiara Hospital, Pisa,
2 Institute of Rheumatology,
University of Siena, Siena,
3 Orthopedic Institute Piero Palagi, Florence, Italy,
4 PAS, Bonn, Germany and
5 Prodesfarma Research Centre, Barcelona, Spain
Received: 3rd July 1995
INDEX
Summary
A long term multi-centre, double-blind, parallel group study was undertaken to investigate the efficacy and safety of aceclofenac (170 patients, 100 mg b.i.d. and placebo once daily) in comparison to diclofenac (173 patients, 50 mg t.i.d.) given for 6 months to patients of both sexes with active rheumatoid arthritis. Efficacy was evaluated in 131 aceclofenac and 130 diclofenac patients at 15 days, 1, 2, 4 and 6 months. Although both treatment groups showed significant improvement in all evaluations of pain and inflammation (assessed by a Visual Analogue Scale and the Ritchie Index) and a progressive reduction in morning stiffness, there were no significant differences between the groups. There was, however, a trend towards greater improvement in hand grip strength with aceclofenac (22% improvement) than diclofenac (17% improvement). Adverse events in both groups were minor, predominantly gastro-intestinal, and fewer patients tended to experience gastro-intestinal events on aceclofenac (13%) than on diclofenac (17%). The overall assessment of tolerance, however, did not differ significantly between groups. In summary, this study supports a therapeutic role for aceclofenac in the treatment of rheumatoid arthritis, and suggests it is an effective and safe NSAID for the treatment of this disease.
Key Words: Aceclofenac - diclofenac - non-steroidal anti-inflammatory drugs - rheumatoid arthritis - pain - inflammation
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease of the joint space which involves synovial proliferation and cartilage destruction. Non-steroidal anti-inflammatory drugs (NSAIDs) are considered to be the first-line symptomatic treatment for RA12. Since most patients require chronic NSAID therapy it is important for an NSAID to be well tolerated in addition to being effective. Indeed, the threat of serious gastro-intestinal complications is a major concern of long-term NSAID therapy3.
Aceclofenac is a new NSAID of the phenylacetic acid class of NSAIDs (2-[(2,6-dichlorophenyl)amino]phenylacetoxyacetic acid), which has been shown to exhibit good analgesic, antipyretic and anti-inflammatory efficacy in several animal models of acute and chronic inflammation9. Although maintaining a similar pharmacodynamic profile to that of indomethacin and diclofenac, aceclofenac has shown better gastric tolerance when compared to other NSAIDs8. In rodents, the acute gastric ulcerogenic activity of aceclofenac was found to be 2-, 4- and 7-fold less than naproxen, diclofenac or indomethacin, respectively. Indeed the therapeutic index for aceclofenac was reported to be four times greater than that of diclofenac, a well-established NSAID in clinical use.
Short-term clinical studies have demonstrated the efficacy of aceclofenac in pain relief following dental extraction and episiotomy 4, 16, and in the chronic treatment of rheumatoid arthritis2 and osteoarthritis5. Furthermore, two studies have demonstrated that aceclofenac is at least as effective as ketoprofen and better tolerated, in terms of fewer drop-outs, in the long-term treatment of RA7,10. In some comparative studies in joint diseases, there was a tendency for aceclofenac to be better tolerated than diclofenac5, 11 or ketoprofen10, with fewer patients being withdrawn from treatment due to gastric intolerance.
This paper presents the results of a 6-month, double-blind, parallel group evaluation of the safety and efficacy of aceclofenac in comparison to the established NSAID diclofenac17 in patients with RA.
Methods
Study population and study design
The study was planned as a multicentre, double-blind, randomized, parallel group investigation of the efficacy and safety of aceclofenac in comparison to diclofenac given for 6 months to patients with active RA. The study was performed in 19 centres in Italy. Patients of either sex (age 20-65 years) with confirmed active RA, according to the American Rheumatism Association diagnostic criteria1 were considered eligible for the study. Active disease was diagnosed by the presence of at least three of the following: swollen joints (at least three); spontaneous pain and sensitivity to pressure in joints (at least 6); morning stiffness (at least 45 minutes) and/or an erythrocyte sedimentation rate (ESR) of > 28 mm/hour, together with a negative test for faecal occult blood. Patients were excluded if they had a history of non-RA arthritic or muscular disease, gastro-intestinal, renal, hepatic or cardiovascular disease. Other exclusion criteria included treatment with anticoagulant drugs, previous hypersensitivity to any NSAID, treatment with unspecified disease-modifying antirheumatic drugs and treatment with other drugs which may interfere in the evaluation of the study drugs in the previous 4 weeks. Pregnant or breast-feeding women and patients on hormonal contraception were not eligible for the study.Ethical committee approval was obtained from the European Ethical Review Committee of Leuven, the Italian Ministry of Health and from the appropriate local committees in all 19 participating centres. The study was conducted in accordance with the European Good Clinical Practice Guidelines. Written informed consent was obtained from all eligible patients prior to entry. A sample size of 140 patients per treatment group was considered sufficient to detect a difference in incidence of side-effects between groups of 10% at the 5% significance level with a power of 85-95%.
Medication
All eligible patients were allocated to one of the two treatment groups according to a randomization schedule: group A received aceclofenac tablets (100 mg b.i.d.) and group B received diclofenac (50 mg t.i.d.). A placebo tablet was introduced into the aceclofenac group to comply with the double-blind conditions.
Study schedule
The initial screening assessment (visit 1) consisted of a full medical history including clinical verification of the disease. Blood and urine samples were taken for routine laboratory screens which included full blood count, plasma proteins, ESR, CRP, renal and hepatic tests. Baseline evaluations were carried out at visit 2 and eligible patients, who satisfied all selection criteria, were randomly assigned to double-blind treatment and medication was dispensed. Clinical efficacy assessments, including measurement of vital signs and laboratory blood tests, were undertaken at 15 days after the start of the study and at 1, 2, 4 and 6 months, when the final evaluation was undertaken.
Clinical efficacy and safety assessments
Clinical efficacy was determined by the following parameters: assessment of pain (using a 100 mm visual analogue scale (VAS): 0 = no pain, 100 = maximum pain); assessment of joint inflammation (tenderness on pressure or movement) using the Ritchie Index
18, the duration of morning stiffness (minutes) and hand grip strength measured by dynamometer (mean of 3 recordings per hand in mmHg). The nature of any adverse events and the possible relationship to treatment was recorded at each visit together with any concomitant and intercurrent diseases, and a count of returned tablets was made to check compliance.Both the investigator and patient made an overall assessment of efficacy and tolerability at the end of the study.
Statistics
All patients randomized to treatment were eligible for an intention to treat analysis of safety. Efficacy was assessed on the basis of an analysis of patients completing the study. Comparability of treatment groups was analysed using chi-square tests for the analysis of categorical baseline parameters and a two-way analysis of variance (ANOVA split-plot) for continuous characteristics. Changes in continuous efficacy variables over time were tested within each group using the ANOVA split-plot and the t-test for differences between groups. Differences in overall assessment by the physician and patients and the incidence of adverse events between groups were compared using either chi-square tests or Mann-Whitney U-tests, as appropriate. A probability of 0.05 was set as the minimum level of significance. Data are given as numbers of patients or means ± SD. All data analyses were performed using SPSS software.
Results
Patient population
Three hundred and forty-three patients were enrolled in the study, of whom 170 received aceclofenac 100 mg b.i.d. with placebo and 173 received diclofenac 50 mg t.i.d. Of the 343 eligible patients, 16 patients (8 aceclofenac patients and 8 diclofenac patients) were excluded from data analysis owing to violation of the protocol. Thus 162 patients in the aceclofenac group and 165 patients in the diclofenac group were evaluated following completion of the study.
The demographic parameters, as summarized in Table I were comparable in each group. Assessment of baseline clinical parameters indicated significantly higher scores in both the Ritchie Index and in the VAS pain rating within the aceclofenac group in comparison to the diclofenac group. However, when taking into consideration the 131 aceclofenac and 130 diclofenac patients who completed the 6 month study, baseline values in these patients only differed significantly for the Ritchie Index (Table II). All other baseline assessments (laboratory tests and vital signs) were comparable in each group (data not shown).
Table I.
Demographic and baseline characteristics of patients enrolled. Values are numbers or
means ± S.D.
| Aceclofenac (n = 162) | Diclofenac (n = 165) | |
| Ratio male : female | 28 : 134 | 33 : 132 |
| Age (years) | 50.5 ± 11.6 | 51.0 ± 10.6 |
| Disease onset (months) | 70.2 ± 62.6 | 72.8 ± 77.8 |
| Pain, VAS (mm) | 63.0 ± 18.0* | 58.1 ± 19.6 |
| Morning stiffness (min) | 82.6 ± 44.4 | 85.6 ± 45.9 |
| Ritchie Index | 23.3 ± 11.5* | 20.6 ± 10.4 |
| Handgrip (mmHg) | 78.8 ± 42.4 | 80.8 ± 43.7 |
Table II.
Baseline and final values for efficacy parameters in patients who completed the study.
Values are means ± S.D.
| Parameter | Aceclofenac | (n = 131) | Diclofenac | (n = 130) |
| Baseline | 6 months | Baseline | 6 months | |
| Pain, VAS (mm) | 62.0 ± 17.0 | 37.9 ± 18.9** | 57.6 ± 19.5 | 36.8 ± 20.0** |
| Morning stiffness (min) | 81.3 ± 42.6 | 48.7 ± 39.0** | 84.9 ± 45.4 | 46.0 ± 39.1** |
| Ritchie Index | 22.4 ± 11.5a | 12.3 ± 8.0** | 20.0 ± 10.3 | 11.8 ± 7.9** |
| Handgrip (mmHg) | 74.6 ± 41.7 | 91.2 ± 41.8** | 80.9 ± 42.8 | 95.0 ± 43.8** |
**p <0.01 vs baselineap
*p <0.05 vs diclofenac
A total of 66 patients, 31 in the aceclofenac group and 35 in the diclofenac group, were excluded from the 6 month efficacy analysis for the following reasons: non-compliance due to inefficacy of treatment (12 aceclofenac, 15 diclofenac); adverse events (12 aceclofenac, 13 diclofenac); surgery and non-compliance due to subjective reasons (7 aceclofenac, 7 diclofenac). Thus, in summary, 131 aceclofenac patients and 130 diclofenac patients were evaluated for efficacy of treatment while 162 aceclofenac and 165 diclofenac patients were evaluated for their tolerance to treatment.
Efficacy
The degree of pain, as measured by the VAS, decreased continually in both groups during the treatment (Figure 1). Statistically significant improvement in comparison to baseline was observed with both aceclofenac and diclofenac already after 15 days and this improvement was maintained for the whole 6 month period (Table II). However, only after 1 month was a statistically significant difference detectable between aceclofenac and diclofenac (Figure 1).

Figure 1.
Improvement in pain assessment on the VAS. Values are means.
All values are significantly different from the respective baseline values (**p <0.01). (a) Significantly from the respective diclofenac value at 1 month ( p < 0.05)
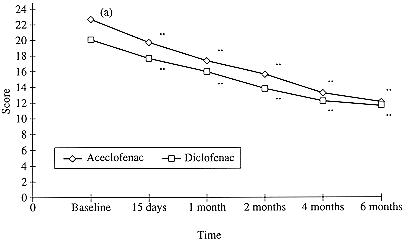
Figure 2.
Improvement in the Ritchie Index. Values are means.
All values are significantly different from the respective baseline values (**p <0.01). (a) Significantly different from the respective diclofenac value at baseline ( p < 0.05)
Joint inflammation, as assessed by the Ritchie Index, also decreased continuously in both groups in comparison to baseline, achieving statistical significance after 15 days which was maintained over the 6 month treatment period (Figure 2 , Table II). However, in these patients who all completed the study, the baseline Ritchie Index was already significantly greater (p <0.05) in the aceclofenac than in the diclofenac group, indicating that the improvement in the aceclofenac group was achieved from a more severe starting point than in the diclofenac group.
A similar progressive reduction in morning stiffness was noted in both groups with a statistically significant difference against baseline values at all time points (Figure 3; Table II). There were no differences between groups for this parameter. In addition, hand grip strength improved from 1 month of treatment in both treatment groups (Figure 4), with a 22% improvement in the aceclofenac group as compared to a 17% improvement in the diclofenac group at completion of the study (Table II). Nevertheless, differences between groups failed to reach statistical significance.

Figure 3.
Improvement in morning stiffness. Values are means.All values are significantly different from the respective baseline values (*p <0.05; **p < 0.01)
The overall assessment of efficacy by the patient and investigator did not reveal any significant differences between groups. Efficacy was assessed as good to very good in 109 of 155 cases (70.3%) by patients and in 119 of 156 cases (76.3%) by investigators in the aceclofenac group and in 103 of 157 cases (65.6%) by patients and in 110 of 158 cases (69.6%) by investigators in the diclofenac group.

Figure 4.
Improvement in hand grip strength. Values are means.
All values from 1 month onwards are significantly different from the respective baseline values (**p <0.01)
Safety and adverse events
During treatment, 42 of 162 patients (25.9%) in the aceclofenac group and 44 of 165 patients (26.6%) in the diclofenac group reported adverse events. Withdrawals due to adverse events which were considered at least possibly related to the study medication are listed in Table III. In total, 21 patients (13.0%) in the aceclofenac group suffered from gastro-intestinal intolerance which caused discontinuance of treatment in 6 cases, as compared to 28 diclofenac patients (17.0%) who suffered gastro-intestinal symptoms, of whom 8 were withdrawn from the study. The overall assessment of tolerance, however, did not differ significantly between groups.Discussion
This long-term comparative study of aceclofenac and diclofenac in RA has confirmed that the therapeutic efficacy of aceclofenac is comparable to that of diclofenac, a well-established NSAID17, in the treatment of rheumatoid arthritis. During the study, both drugs improved the degree of pain, as indicated by the VAS and the Ritchie Index. The fact that, at baseline, both these variables were significantly greater in the aceclofenac than in the diclofenac group suggests that the RA disease may have been more severe in the aceclofenac group and that the efficacy of this drug may have been somewhat greater, since there was no difference between the groups after 6 months. This possibility is supported by the fact that aceclofenac tended to be more effective than diclofenac in improving the Ritchie Index and grip strength. In a previous 3 month study in RA, aceclofenac was found to be significantly more effective than ketoprofen, both with regard to the Ritchie Index and morning stiffness10.
Table III.
Number of study withdrawals due to adverse events considered at least possibly related to study medication
| Adverse events* | Aceclofenac (n = 162) | Diclofenac (n = 165) |
| Gastro-intestinalGastralgia | 3 | 3 |
| Epigastralgia | 2 | 4 |
| Gastric pyrosis | 1 | 2 |
| Diarrhoea | 1 | 1 |
| Abdominal pain | 1 | 1 |
| Melena | 1 | - |
| Occult blood in faeces | 1 | - |
| Dyspepsia | - | 1 |
| HepaticRaised enzymes | 1 | 3 |
| SkinPruritis | 1 | 1 |
| Erythema | 1 | - |
| RenalNitrogen | - | 1 |
| Haematuria | - | 1 |
| OtherVertigo | 1 | 1 |
| Dyspnoea | 1 | - |
| Haematocrit/Hb | 1 | - |
| Asthenia | - | 1 |
| Total number of withdrawals | 12 (7.4%) | 13 (7.9%) |
The main disadvantage of long-term therapy with NSAIDs is the risk of gastro-intestinal disturbances. NSAIDs carry a greater risk of inducing upper gastro-intestinal bleeding than simple analgesics15, though the risk is dependent on the dose and is highest in patients who have previously suffered bleeding episodes6. Ibuprofen and diclofenac have recently been shown to be the NSAIDs, of those most frequently used, which have the lowest risk of causing upper gastro-intestinal bleeding13, 14. Any NSAID which, therefore, has comparable or better gastro-intestinal tolerability than these two drugs is likely to be well accepted for long-term therapy of RA. Preclinical studies demonstrated that aceclofenac not only has a superior therapeutic activity but also a more favourable ulcerogenic index than diclofenac, phenylbutazone or indomethacin8. Furthermore, results of previous comparative clinical trials with ketoprofen showed that aceclofenac, while being similar in terms of therapeutic efficacy, demonstrates a better safety and tolerability profile 7, 10. In the present study, aceclofenac was also well tolerated by patients, the incidence of gastro-intestinal intolerance tending to be lower in the aceclofenac group (13%) than in the diclofenac group (17%). These findings not only confirm the results of previous studies in RA demonstrating the good tolerability of the drug2, 7, 10, they also support earlier findings in healthy volunteers showing that aceclofenac tended to be more tolerable than diclofenac in terms of gastro-intestinal blood loss 19.
In conclusion, the findings described here confirm the good safety profile of aceclofenac and, together with its sustained analgesic efficacy, indicate that it may be considered as an effective and well-tolerated NSAID for the long-term treatment of RA.
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages copyright ©Priory Lodge Education Ltd 1994-

