Risk factor model for developing postpartum diabetes mellitus in patients with Gestational Diabetes Mellitus (GDM)
James C McElnay (Professor) and Asim A Elnour (Ph.D)
J.C. McElnay1and A.A. Elnour1,2
1 Clinical and Practice Research Group, School of Pharmacy, Queen's University Belfast, Belfast, UK.
Title: Dean Faculty of Medicine, Queen's University Belfast, Belfast, UK.
2 Clinical Pharmacy Unit, Al Ain Hospital, General Health Services For AbuDhabi Emirate, Eastern Region, Al Ain, (UAE).
Title: Head of Clinical Pharmacy Unit
Abstract
Aims and Objectives To determine the prevalence of postpartum diabetes in a sample of UAE women who suffered from Gestational Diabetes Mellitus (GDM). To examine factors, obtained from patient charts and at interview, with regard to their involvement in placing a patient at increased risk of developing diabetes mellitus and can predict the risk of overt diabetes mellitus. A further aim was to develop a risk factor model (using logistic regression analysis) that delineates factors implicated in putting these GDM patients at increased risk of developing diabetes mellitus during the postpartum period.
Setting: The antenatal and postnatal wards in Al Ain Hospital, UAE.
Methods All women who were entered into the study (n=165) were diagnosed with GDM in the Al Ain Hospital, UAE, during the period from December 2001 to June 2002. The initial screening was performed using a 50 g OGTT and the diagnosis of GDM was made (100 g OGTT) according to the criteria outlined by Coustan and Carpenter (1982). All patients underwent postpartum glucose tolerance testing (75 g OGTT) within six to twelve weeks after delivery. The diagnosis of postpartum diabetes was confirmed with the criteria described by the Expert Committee on the Diagnosis and classification of Diabetes Mellitus. All patients were followed up until delivery and for six months during the postpartum period.
Multivariate logistic regression analysis yielded a four-variable predictive model. This final predictive model had a specificity of 78.6%, sensitivity of 58.8% and overall accuracy of 74.5 (cut-off point of 0.25).
Key findings: Risk model developing postpartum diabetes in GDM patients.
Conclusions GDM in the Al Ain-UAE, is recognised as a very strong predictor of later diabetes mellitus, particularly type-II. The risk factors identified were family history of diabetes mellitus (OR 5.51), severe hyperglycaemia (OR 4.62), gestational age at GDM diagnosis <16 weeks (OR 3.96) and gravida >5 (OR 3.23).
Introduction
It is well established that women with Gestational Diabetes Mellitus (GDM) have a considerable risk of developing diabetes later in life. The metabolic disturbances of GDM put women at increased risk of developing particularly type-II diabetes. Many patients with GDM will be diagnosed as having diabetes type-I, diabetes type-II, impaired fasting glucose (IFG), or impaired glucose tolerance (IGT) at mid and long-term follow-up [1,2,3,4,5,6].
Kjos and associates (1990) evaluated women between the fifth and eighth week postpartum with a 2 hour oral glucose tolerance test (OGTT) and found 81.0% of patients had normal or unclassifiable OGTT results. An additional 10.0% had impaired glucose tolerance and the remaining 9.0% had diabetes mellitus. Forty-four percent of women with fasting glucose levels >7.8 mmol/L during pregnancy had diabetes diagnosed by an OGTT at their initial postpartum visit. These researchers suggest that routine testing should be performed even in patients with a history of diet-controlled GDM, since they found a 2.0% prevalence of diabetes mellitus and an 8.0% prevalence of impaired glucose tolerance in these women [7].
Coustan and associates (1993) studied former gestational diabetic women and found diabetes or impaired glucose tolerance in 6.0% of those tested at 0-2 years, 13.0% at 3-4 years, 15.0% at 5-6 years, and 30.0% at 7-10 years postpartum [8]. Other studies have documented type-II diabetes, 3 to 5 years postpartum in 30.0% - 50.0% of previous GDM women [9]. A postpartum metabolic assessment (3 to 6 months) in women with GDM revealed a 5.4% prevalence of diabetes using the American Diabetes Association (ADA) criteria [10].
In Al Ain (UAE), over a 5 year period, 549 patients underwent the 2 hour, 75 g OGTT, four to eight weeks after delivery. They were classified by the criteria of WHO , the ADA and the revised WHO criteria [11,12,13]. The prevalence of diabetes by WHO and ADA criteria were similar (8.2% vs. 6.6%) but estimates of impaired glucose homeostasis varied widely (15.5% impaired glucose tolerance vs. 9.3% impaired fasting glucose, respectively; [14].
Review of many worldwide studies of diabetes among former GDM patients has therefore indicated a wide range of incidence rates, from 10.0% to 87.0% for combined diabetes mellitus and impaired glucose tolerance and 5.4% to 62.0% for diabetes mellitus. The varying observation periods and the differing classification criteria used in different studies is obviously a factor in this variability.
The postpartum follow-up of women with GDM is crucial to monitor if normal glycaemia returns after delivery. All women with GDM should also receive contraceptive advice and counselling regarding future pregnancies. Some studies have demonstrated that postpartum diabetes education, especially for high risk women, may prevent or delay the development of type-II diabetes and its sequellae [15]. Women with a history of GDM therefore comprise an ideal group of patients to implement early interventional measures to halt the progression to diabetes [16].
One of the most recent controversies concerns the criteria that should be used for postpartum screening for diabetes mellitus in previous GDM patients. Some studies have suggested the use of WHO criteria [17] while other studies have used the criteria established by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (ECDCDM) [18,19]. Furthermore, some studies have used the ADA criteria, which include the term impaired fasting plasma glucose [20].
In one study for the classification of glucose intolerance in women with previous GDM (1 to 86 months after delivery) by the WHO [11] criteria and the ADA [12] criteria, have provided estimates of diabetes prevalence of 13.3% vs. 11.5% respectively [21]. The rate of postpartum abnormalities in glucose metabolism more than doubles when the ADA criteria are applied (as compared with National Diabetes Data Group), i.e. more women are identified with lesser degrees of impairment. However, relying on fasting glucose levels alone, without glucose tolerance testing, may miss one third of women with such abnormalities [22]. Recently it has been found that fasting plasma glucose is an unsatisfactory method of evaluating the glucose tolerance of Caucasian women with previous GDM and OGTT use has been suggested as a better method for such a purpose [23,24].
The frequency of postpartum glucose intolerance among Indo-Asian women is significantly greater than among age-matched Caucasian and Afro-Caribbean women. It has been suggested that all women with gestational diabetes should undergo postpartum screening for persistent glucose intolerance.
This later study measured the prevalence of persistent glucose intolerance at six to twelve weeks postpartum in various ethnic groups to assess the value of targeted postpartum screening. The study showed that 35.0% of Indo-Asians had persistent postpartum glucose intolerance compared with 7.0% of Caucasians and 5.0% of Afro-Caribbean’s (p< 0.003). The authors concluded that insulin requirement during pregnancy and a diagnosis of gestational diabetes prior to 20 weeks of pregnancy were predictive for persistent postpartum glucose intolerance amongst Indo-Asians [25].
The various guidelines for postpartum follow-up of GDM produce similar estimates for diabetes prevalence but widely discordant results for glucose intolerance. Until more uniform evidence-based criteria become available, the various strategies for GDM follow-up will continue to cause confusion in clinical practice. Screening for diabetes can be performed at the six week postpartum visit.
The ADA recommended that reclassification of maternal glycaemia status should be performed within six weeks after delivery and according to the guidelines of the "Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus". If glucose levels are normal postpartum, reassessment of glycaemia should be undertaken at a minimum of three year intervals.
Methods and Study Design
The study was a randomised, controlled, longitudinal, prospective clinical trial. The study was approved by the Research Ethics Committee (RECA/01/26), Faculty of Medicine, Emirates University in the Al Ain, UAE. The study site was Al Ain Hospital, UAE. The hospital is well-equipped 450-bed facility, which serves as a teaching site for the medical students of the Faculty of Medicine, Emirates University.
All patients were followed up until delivery and for six months during the postpartum period. All patients underwent postpartum glucose tolerance testing (75 g OGTT) within six to twelve weeks after delivery. The diagnosis of postpartum diabetes was confirmed with the criteria described by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus.
Medical and demographic information regarding each patient was collected via a medical chart review. In addition each patient was interviewed to obtain further relevant information, using a questionnaire developed for the study of diabetes in pregnancy. Patients were interviewed at an outpatient clinic and/or during their hospital stay. During the interview patients were also asked about their past medical history. Patient data from the chart review and the interview were entered into SPSS for analysis.
The recommendations of Hosmer and Lemshow [26] were used to identify the factors putting GDM patients at increased risk of developing postpartum diabetes and to build risk factor models for postpartum diabetes.
Data analysis
An analysis for the association of the risk factors and postpartum diabetes was conducted as follows:
Chi-squared analysis (χ2) risk estimation of odds ratios (OR) relating to possible risk factors in patients dichotomised as having a positive diagnosis of diabetes mellitus postpartum or not.
Logistic regression analysis - binary logistic (Hosmer Lemshow goodness-of fit) by backward conditional method.
Development of risk factor model
The following procedures were used to identify the factors putting GDM patients at increased risk of developing postpartum diabetes and to build risk factor models for postpartum diabetes according to the recommendations of Hosmer and Lemshow [26].
1. Application of a recognised statistical method to produce statistically relevant risk variables (Chi-squared analysis).
2. Each variable (if present in at least 5% of population) with a p value <0.10 was considered a candidate for logistic regression modelling.
3. The candidate variables identified as detailed above from patient chart reviews (maternal and neonatal charts) and patient interviews were entered separately into a stepwise backward elimination logistic regression analysis with model entry set at p= 0.2 and model removal set at 0.2 as recommended by Hosmer and Lemshow [27], to produce a multivariate model, one for chart data and the second for interview data.
4. The two datasets were considered separately at this stage due to the large number of variables under consideration and taking account of the recommendation of Hosmer and Lemshow [27] regarding the number of variables entered into a logistic regression when compared with the number of subjects involved.
5. The procedure (outlined in point 3) was repeated for entry and removal values of p= 0.15, 0.1 and 0.05.
6. The significant explanatory variables contained in the separate models (chart and interview) were then combined and re-examined using further logistic modelling.
7. For those variables which remained in the final model, an examination of their significance (p< 0.05) was undertaken to evaluate their contribution to the final model.
8. A range of different cut point values were assessed to allow the selection of the best one. The cut-off point for risk assessment was set at 0.25 i.e. above this point a patient was considered to be at high risk of postpartum diabetes mellitus.
9. To check that all variables were independent, the phi-co-efficient was used.
10. A receiver-operator characteristics (ROC) curve was constructed after applying the final refined predictive model to the study population.
Results
Data analysis revealed that the number of patients who developed postpartum diabetes mellitus was 34, i.e. a prevalence of 20.6% from the total study population (Table 1 and figure 1). The univariate analysis (Chi-squared χ2) analysis indicated that a number of variables from both chart review and interview data (32 variables), were statistically related (p< 0.10) to the development of postpartum diabetes mellitus.
Table 1 Incidence of postpartum diabetes in study population (p= 0.012)
Study patients |
Postpartum diabetes mellitus |
Total |
|
No |
Yes |
||
Number of patients |
131 79.4% |
34 20.6% |
165
|
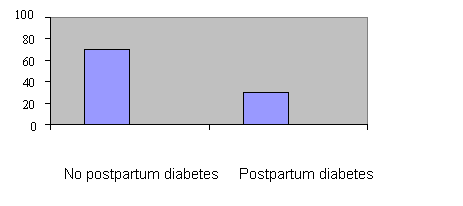
Figure 1 The incidence (%) of postpartum diabetes mellitus in the GDM
population (n=165)
Using stepwise backward logistic regression analysis, any variable whose univariate test had a p-value of <0.10 was a candidate for multivariate modelling. The total number of variables with a p-value of <0.10 from the Chi-squared tests (likelihood ratio test statistics) was 18 variables (Table 2).
Table 2 Variables significantly related (p< 0.10) to the development of postpartum diabetes
Variable
|
OR |
95% CI |
p value |
Hydramnios
|
4.17 |
1.47 – 11.82 |
0.009 |
Severe hyperglycaemia (fasting > 6.7 mmol/L and/or 1 hour postprandial >7.8 mmol/L) |
3.92 |
1.47 – 10.47 |
0.008 |
Obstructed labour
|
8.60 |
1.50 – 49.15 |
0.017 |
Need for caesarean section
|
4.35 |
1.60 – 11.81 |
0.005 |
Neonatal hypoglycaemia (< 2.6 mmol/L)
|
5.47 |
1.38 – 21.65 |
0.019 |
Macrosomia
|
2.79 |
1.14 – 6.84 |
0.035 |
Large for gestational age
|
2.78 |
1.09 – 7.07 |
0.052 |
Admission to special care baby unit (within the first 48 hours) |
2.85 |
1.31 – 6.20 |
0.012 |
Pre-eclampsia toxicity
|
1.26 |
1.17 – 6.93 |
0.061 |
Frequent urinary tract infection (more than 3 times per month) |
4.35 |
1.60 – 11.81 |
0.005 |
History of GDM
|
2.99 |
1.21 – 7.39 |
0.030 |
History of abortion
|
4.73 |
1.62 – 13.75 |
0.060 |
Family history of GDM
|
4.17 |
1.47 – 11.82 |
0.009 |
Family history of DM
|
3.35 |
1.22 – 9.16 |
0.029 |
Insulin need during the index pregnancy
|
2.29 |
1.01 – 5.17 |
0.053 |
Age group <29
|
2.53 |
1.15 – 5.55 |
0.021 |
Gravida >5
|
2.65 |
1.22 – 5.73 |
0.015 |
Gestational age at diagnosis <16 weeks
|
3.77 |
1.63 – 8.69 |
0.021 |
Key: OR = Odds Ratios (Exp B); CI = Confidence Intervals.
reference to the total number of patients in the study population (165 patients), the number of patients with postpartum diabetes mellitus was 34, and the number of patients with no postpartum diabetes was 131. As per the recommendation of Hosmer and Lemshow [26], the rule of ten was followed which suggests a final predictive model with 4 variables (i.e. approximately one tenth of the smaller group of patients).
Entering of the significantly associated variables (from patient chart or patient interview independently) into stepwise backward elimination logistic regression analysis with model entry set at p=0.20, and model removal set at p=0.20 [27] yielded two separate preliminary models for chart and interview data. Repeating this procedure for further elimination with entry and removal values of p=0.15, p=0.10 and p=0.05 yielded final models for both the patient chart and the patient interview data.
The final chart model included five statistically significant variables (p value < 0.05), the specificity of the model was 84.7%, the sensitivity was 52.9% and the overall accuracy was 78.2%. The Hosmer and Lemshow goodness-of-fit test gave a Chi-squared value of 0.627 (3 degrees of freedom, p=0.890) i.e. not significant; therefore the model can be regarded as having acceptable fit to the data used (Table 3).
Table 3 Final logistic model derived from patient chart review
|
Variable |
B
|
OR |
95% CI |
p value |
|
Severe hyperglycaemia (fasting >6.7 mmol/L and/or 1 hour postprandial >7.8 mmol/L) |
1.33 |
3.79 |
1.26 - 11.47 |
0.010 |
|
Obstructed labour
|
2.16 |
8.70 |
1.28 - 59.24 |
0.027 |
|
Need for caesarean section
|
1.26 |
3.52 |
1.15 - 10.73 |
0.027 |
|
Neonatal hypoglycaemia (< 2.6 mmol/L) |
2.03 |
7.57 |
1.76 - 132.52 |
0.006 |
|
Admission to special care baby unit (within the first 48 hours) |
1.08 |
2.93 |
1.23 - 6.98 |
0.015 |
The final model derived from the patient interview data included seven statistically significant variables. The specificity of the model was 77.9%, the sensitivity was 79.4% and the overall accuracy was 78.2%. The Hosmer and Lemshow goodness-of-fit test gave a Chi-squared value of 5.82 (7 degrees of freedom, p= 0.561) i.e. not significant; therefore the model can be regarded as having acceptable fit to the data used (Table 4). The number of significant variables from the resulting models (the chart review and interview models) was therefore twelve. A further regression was performed to allow the two individual models to be combined with model entry and removal set this time at p= 0.02. A final predictive model for developing postpartum diabetes mellitus with four variables was produced (Table 5).
The cut-off point for risk assessment was set at 0.25 i.e. above this point a patient is considered to be at high risk of developing diabetes mellitus. With this cut-off point the specificity of this model was 78.6%, the sensitivity was 58.8% and the overall accuracy was 74.5%, (Table 6).
Table 4: Final logistic model derived from patient interview data
|
Variable |
B
|
OR |
95% CI |
p value |
|
History of GDM
|
1.86 |
6.39 |
1.96 - 20.90 |
0.026 |
|
Family history of diabetes mellitus
|
1.42 |
4.13 |
1.43 - 12.02 |
0.007 |
|
History of abortion
|
2.08 |
8.02 |
2.11 - 30.45 |
0.020 |
|
Family history of GDM
|
1.46 |
4.29 |
1.19 - 15.48 |
0.029 |
|
Insulin use in the index pregnancy
|
0.99 |
2.68 |
1.00 - 7.21 |
0.050 |
|
Gravida >5
|
1.27 |
3.55 |
1.37 - 9.27 |
0.009 |
|
Gestational age at diagnosis <16 weeks |
1.32 |
3.75 |
1.34 - 10.49 |
0.012 |
Key: B = Variable coefficient, OR = Odds Ratios (Exp B) and CI = Confidence Intervals
Table 5 The final logistic regression model for developing postpartum diabetes mellitus in current GDM patients at the Al Ain Hospital, UAE
Variable |
B
|
OR |
95% CI |
p value |
Severe hyperglycaemia (fasting >6.7 mmol/L and/or 1 hour postprandial >7.8 mmol/L) |
1.53 |
4.62 |
1.49 – 14.34 |
0.008 |
Family history of diabetes mellitus
|
1.31 |
5.51 |
1.71 – 17.80 |
0.004 |
Gravida >5
|
1.17 |
3.23 |
1.36 – 07.63 |
0.008 |
Gestational age at diagnosis <16 weeks
|
1.38 |
3.96 |
1.51 – 10.35 |
0.005 |
Table 6 Observed and predicted totals, sensitivities and specificities from final logistic regression model for developing postpartum diabetes mellitus in current GDM patients
|
|
Observed Yes/No |
Row totals |
Percentage correct (%) |
|
Test Yes |
20 |
48 |
78.6 Specificity |
|
Prediction No |
14 |
117 |
58.8 Sensitivity |
|
Column total |
34 |
165 |
74.5 Accuracy |
Calculation key:
Sensitivity = 20/34 = 58.8
Specificity = 103/131 = 78.6
% correct (Accuracy) = 20+103/165 = 74.5
The Hosmer and Lemshow goodness-of-fit test gave a Chi-squared value of 3.18 (4 degrees of freedom, p= 0.528); therefore the model can be regarded as having acceptable fit to the data used. To check that all variables were independent, the phi-co-efficient test was calculated. The phi-co-efficient generated from each pair of variables were <0.07, therefore the variables in the final model can be regarded as independent.
A ROC curve was constructed after applying the final predictive model to the study population (165 patients). The area under the curve (AUC) was equal to 0.764, thus demonstrating that the final postpartum diabetes predictive model can effectively discriminate false and true positives across all thresholds of risk (see figure 1.2). True Negative: a negative test result for an individual that is truly negative for developing postpartum diabetes. True Positive: a positive test result for an individual that is truly positive for developing postpartum diabetes. Larger values of the AUC value indicate stronger evidence for a positive actual state (the positive actual state is 1.00 for developing postpartum diabetes mellitus) i.e. the closer the predicted value of AUC approaches the actual status 1.00, the stronger the model will be in predicting the actual true status of developing postpartum diabetes mellitus.
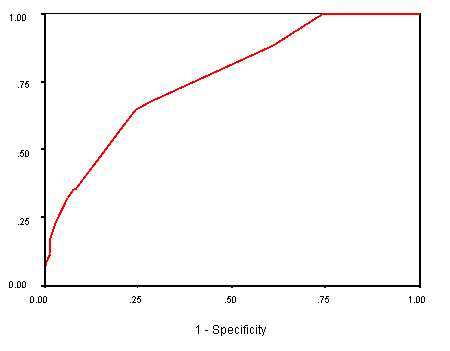
Figure 2 Receiver-operating characteristics (ROC) curve for final postpartum
diabetes mellitus risk model [area under the curve (AUC) = 0.764]
Discussion
The risk of developing diabetes immediately postpartum in GDM patients in the UAE, has neither to not been fully examined. The prevalence of postpartum diabetes in the current GDM patients was found to be 20.6%. The incidence of postpartum diabetes in the present study population was therefore higher than that previously reported in similar studies [7,15].
These later studies documented a 9.0% - 6.3% prevalence of postpartum diabetes mellitus (five to eight weeks and six to twelve weeks postpartum, respectively) in previous GDM patients with a 2 hour OGTT. This difference may, however, be attributed to the different criteria used for diagnosing GDM as well as for diagnosing postpartum diabetes. These later studies used the WHO criteria for the classification of postpartum diabetes in previous GDM patients, whereas, the present study used the criteria established by the ECDCDM.
However, a similar study to the present research work demonstrated a prevalence of 15.1% of postpartum diabetes in an Asian population [17]. In the later study GDM Korean women were reclassified six to eight weeks after delivery. Furthermore, another similar study conducted in an Indo-Asian GDM population showed that 35.0% of those patients developed persistent postpartum glucose intolerance [25]. However, the authors did not provide more data on, for example, the exact percentage of those who developed overt postpartum diabetes mellitus.
It should be noted that insulin requirement during pregnancy was also found to be related to the development of postpartum diabetes. This finding is in agreement with recently published research [25].
The final risk model included four major independent risk factors. These are discussed below:
(a) Family history of diabetes mellitus (OR 5.51)
Women with a family history of diabetes mellitus (in their first degree relatives) were found to be at high risk of development of postpartum diabetes mellitus. This finding was also consistent with the findings of other researchers [28,29]. Women with a family history of diabetes mellitus therefore comprise a target group for aggressive intervention with the aim to prevent or delay the development of diabetes mellitus.
(b) Severe hyperglycaemia (OR 4.62)
The present study documented a significant association between severe hyperglycaemia during pregnancy and the development of postpartum diabetes mellitus. In this regard the present study is in agreement with recent research findings [30,31,32,33,34]. This finding highlights the importance of identifying GDM patients with frequent severe hyperglycaemia in order to develop strategies for early intervention to prevent and or delay the progression to diabetes mellitus and its sequellae.
(c) Gestational age at GDM diagnosis <16 weeks (OR 3.96)
Previous studies have demonstrated that gestational age <20 and/or <24 weeks is a strong predictor of the development of postpartum diabetes (Buchannan et al., 1998; Dalfra et al., 2001;[6,15,17,25,31]. However, in the present study all patients were recruited at a gestational age of <20 weeks. The gestational age cut-off of <16 weeks was used in the present study as it was the mid point between the highest gestational week (20 weeks) and the lowest gestational age (12 weeks) at the time of patient recruitment.
(d) Gravida (OR 3.23)
GDM patients in the UAE with more than five gravida (number of conceptions) were found to be at high risk of developing postpartum diabetes, which again is consistent with previously published data [15,35,36,37]. Again this has implications for counselling, not only during pregnancy, but also pre-pregnancy.
Several investigators used univariate and multivariate analysisto identify factors associated with conversion of GDM to type-II diabetes.The variables examined varied widely between studies. Althoughmany studies used stepwise logistic regression, it was usuallynot specified how variables were selected or how the model wasconstructed, and the significance of the variables could heavilydepend on the order in which they were entered. The difference in the risk reportedamong more than 28 studies could be explained by different lengths of follow-up,ethnic variation, and the diagnostic criteria used.
Recommendations for postpartum screening should account forthe time elapsed from the index pregnancy and the criteriaused for patient selection. Because women with a family history of diabetes seemed to have the highest future riskof type-II diabetes (OR 5.51), it may be possible to stratify risk furtherbased on this variable.
Conclusions
The risk model developed could help clinical pharmacists and other healthcare providers to focus on a sub-group of GDM patients at increased risk of developing postpartum diabetes.Patients diagnosed with GDM prior to sixteen gestational weeks were more prone to develop diabetes during the postpartum period. Such patients should form a particular target for early intervention.
The research presented supported the recommendation that antenatal OGTT testing during the early second trimester is advisable in high risk populations, in which more women will develop GDM before 20 weeks. Postpartum OGTT is highly recommended to screen for diabetes mellitus, as approximately one fifth of GDM patients will experience overt diabetes in the early postpartum period.
The first OGTT should be performed around two months postpartum in order to diagnose women already with diabetes and to identify women with the highest risk for later development of overt diabetes. Routine testing for the development of diabetes mellitus should be performed even in patients with a history of diet-controlled gestational diabetes, since diabetes mellitus was significantly documented in these women. Diabetes prevention strategies need to address the potentially modifiable risk factors which can promote the development of diabetes mellitus in patients with GDM.
References
1 Greenberg LR, Moore TR, Murphy H. Gestational diabetes mellitus: antenatal variables as predictors of postpartum glucose intolerance. Obstet Gynaecol 1995; 86: 97-101.
2 Fuchtenbusch M, Ferber K, Standl E, Ziegler AG. Prediction of type-I diabetes postpartum in patients with gestational diabetes mellitus by combined islet cell autoantibody screening. Diabetes 1997; 46: 1459-67.
3 King H. Epidemiology of glucose intolerance and gestational diabetes in women of childbearing age. Diabetes Care 1998;21 (Suppl. 2): B9-B13.
4 Herranz L, García-Ingelmo MT, Martín-Vaquero P, Grande C, Pallardo LF. Follow-up of women with gestational diabetes: incidence and factors associated with later development of abnormal glucose tolerance (Abstract). Diabetologia 1998; 41 (Suppl. 1): A125.
5 Jovanovic L, Pettitt D. Gestational diabetes mellitus. JAMA 2001; 286: 2516-18.
6 Schaefer-Graf UM, Buchanan TA, Xiang AH, Peters RK, Kjos SL. Clinical predictors for a high risk for the development of diabetes mellitus in the early puerperium in women with recent gestational diabetes mellitus. Am J Obstet Gynaecol 2002; 186: 751-56.
7 Kjos SL, Buchanan TA, Greenspoon JS, Montoro M, Bernstein GS, Mestman JH. Gestational diabetes mellitus: the prevalence of glucose intolerance and diabetes mellitus in the first two months postpartum. Am J Obstet Gynaecol 1990; 163: 93-8.
8 Coustan DR, Carpenter MW, O'Sullivan PS, Carr SR. Gestational diabetes mellitus: predictors of subsequent disordered glucose metabolism. Am J Obstet Gynaecol 1993; 168: 1139-45.
9 Bian X, Gao P, Xiong X, Xu H, Qian M, Liu S. Risk factors for development of diabetes mellitus in women with a history of gestational diabetes mellitus. Chin Med J Engl 2000; 113: 759-62.
10 Pallardo F, Herranz L, Garcia-Ingelmo T, Grande C, Martin-Vaquero P, Janez M, Gonzalez A. Early postpartum metabolic assessment in women with prior gestational diabetes. Diabetes Care 1999; 22: 1053-58.
11a
World Health Organization. Expert Committee on Diabetes. Technical Report
Series,
13 World Health Organization. Definition, Diagnosis and Classification
of Diabetes Mellitus and its Complications: Report of a WHO consultation.
Part 1: Diagnosis and Classification of Diabetes Mellitus.
14 Agarwal M, Punnose J, Dhatt GS. Gestational diabetes: implications of variation in postpartum follow-up criteria. Eur J Obstet Gynaecol Reprod Biol 2004; 113: 149-53.
15 Larijani B, Hossein-nezhad
A, Pajouhi M, Bastanhagh MH. The predictive factors for gestational diabetes
mellitus and postpartum diabetes mellitus. Endocrinology and Metabolism Research Centre,
16 Tan HH, Tan HK, Lim HS, Tan AS, Lim SC. Gestational diabetes mellitus: a call for systematic tracing. Ann Acad Med Singapore 2002; 31: 281-84.
17 Jang HC, Yim CH, Han KO, Yoon HK, Han IK, Kim MY, Yang JH, Cho NH. Gestational diabetes mellitus in Korea: prevalence and prediction of glucose intolerance at early postpartum. Diabetes Res Clin Pract 2003; 61: 117-24
18 The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183-97.
19 The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003; 26: (Suppl. 1).
20 Buchanan TA, Xiang AH, Kjos SL, Lee WP, Trigo E, Nader I, Bergner EA, Palmer JP, Peters RK. Gestational diabetes: ante partum characteristics that predict postpartum glucose intolerance and type-II diabetes in Latino women. Diabetes 1998; 47: 1302-10.
21 Kousta E, Lawrence NJ, Penny A, Millauer BA, Robinson S, Dornhorst A, de Swiet M, Steer PJ, Grenfell A, Mather HM, Johnston DG, McCarthy MI. Implications of new diagnostic criteria for abnormal glucose homeostasis in women with previous gestational diabetes. Diabetes Care, 1999; 22: 933-37.
22 Conway DL, Langer O. Effects of new criteria for type-II diabetes on the rate of postpartum glucose intolerance in women with gestational diabetes. Am J Obstet Gynaecol 1999; 181: 610-14.
23 Costa A, Carmona F, Martinez-Roman S, Quinto L, Levy I, Conget I. Postpartum reclassification of glucose tolerance in women previously diagnosed with gestational diabetes mellitus. Diabetic Med 2000; 17: 595-8.
24 Cypryk K, Czupryniak L, Wilczynski J, Lewinski A. Diabetes screening after gestational diabetes mellitus: poor performance of fasting plasma glucose. Acta Diabetol 2004; 41: 5-8.
25 Sinha B, Brydon P, Taylor RS, Hollins A, Munro A, Jenkins D, Dunne F. Maternal antenatal parameters as predictors of persistent postnatal glucose intolerance: a comparative study between Afro-Caribbean's, Asians and Caucasians. Diabetic Med 2003; 20: 382-86.
26 Hosmer DW, Lemshow S. Applied logistic regression 2nd
edition
27
Hosmer DW, Lemshow S. Applied logistic regression. 2nd edition
28 Henry OA, Beischer NA. Long-term implications of gestational diabetes for the mother. Baillieres Clin Obstet Gynaecol 1991; 5: 461-83.
29 Grace ME, Rolv S, Lorentz MI. Birth characteristics of women who develop gestational diabetes: population based study. BMJ 2000; 321: 546-47.
30 García-Patterson A, Corcoy R, Balsells M, Altirriba O, Adelantado JM, Cabero LL, de Leiva A. In pregnancies with gestational diabetes mellitus and intensive therapy, perinatal outcome is worse in small for gestational age newborns. Am J Obstet Gynaecol 1998; 179: 481-85.
31 Mercé Albareda, Agueda C, Gemma B, Sandra P, Angels O, Alberto DL, Rosa C. Diabetes and abnormal glucose tolerance in women with previous gestational diabetes. Diabetes Care 2003; 26: 1199-1205.
32 Silva MR, Calderon Ide M, Goncalves LC, Aragon FF, Padovani CR, Pimenta Wde P. Occurrence of diabetes mellitus in women with prior gestational hyperglycaemia. Rev Saude Publica 2003; 37: 345-50.
33 Jovanovic L. Achieving euglycaemia in women with gestational diabetes mellitus: current options for screening, diagnosis and treatment. Drugs 2004; 64: 1401-17.
34 Menato G, Bo S, Gallo ML, Bardelli C, Lezo A, Signorile A, Gambino R, Cassader M, Massobrio M, Pagano G. Mild gestational hyperglycaemia, the metabolic syndrome and adverse neonatal outcomes. Acta Obstet Gyn Scan 2004; 83: 335-40.
35 Damm P, Kuhl C, Bertelsen A, Mölsted-Pedersen L. Predictive factors for the development of diabetes in women with previous gestational diabetes mellitus. Am J Obstet Gynaecol 1992; 167: 607-16.
36 Peters RK, Kjos SL, Xiang A, Buchanan TA. Long-term diabetogenic effect of a single pregnancy in women with previous gestational diabetes mellitus. Lancet 1996; 347: 227-30.
37 Steinhart J, Sugarman J, Connell F. Gestational diabetes is a herald of type-II diabetes in Navajo women. Diabetes Care 1997; 20: 943-47.
38 Buchanan TA, Xiang AH, Kjos SL, Lee WP, Trigo E, Nader I, Bergner EA, Palmer JP, Peters RK. Gestational diabetes: ante partum characteristics that predict postpartum glucose intolerance and type-II diabetes in Latino women. Diabetes 1998; 47: 1302-10.
39 Dalfra MG, Lapolla A, Masin M, Giglia G, Dalla BB, Toniato R, Fedele D. Ante partum and early postpartum predictors of type-II diabetes development in women with gestational diabetes mellitus. Diabetes Metab 2001; 27: 675-80.
40. Coustan DR, Carpenter MW. Criteria for screening tests for gestational diabetes. Am J Obstet Gynaecol 1982; 144: 768-73.
First Published September 2006
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages copyright ©Priory Lodge Education Ltd 1994-

