Once the diagnosis has been established, the aims of treatment are to alleviate symptoms, prevent progression of disease and preserve optimum lung function to improve performance of activities of daily living and enhance quality of life. The European guidelines comment that the pharmacological and rehabilitation therapies that are currently being used in the management of COPD are not truly evidence based and thus a proportion of current practice is empirical. However, this author considers that both sets of treatment guidelines are more evidence based than the equivalent guidelines for the managment of asthma which are used without much question.
Once the diagnosis has been established, both sets of guidelines place great emphasis on the cessation of smoking as one the most important aspects of management. Stopping smoking will slow the rate of lung function decline in patients with COPD as mentioned above. Unfortunately, only about one third of patients, even after extensive counselling, are able to abstain from smoking long term. Factors which encourage continuation of smoking vary from patient to patient, but include the addictive potential of nicotine, conditioned responses to certain situations (work, social or stress related), psychosocial problems, depression, poor education, peer pressure, low income, lack of other stimuli and promotional campaigns by the tobacco companies.
Most smokers will have tried stopping on several occasions with varying degrees of success, and repeated attempts are often required. Smokers need to be constantly encouraged by their physician to go through the cycle of contemplation of cessation, preparation, positive action and maintenance. However, the cycle is usually closed by relapse. The role of the physician is to provide an explanation of the harmful effects of smoking and the benefits of stopping. Then the physician should guide the patient through the various stages and help provide support during the different phases. There are varying levels of support available ranging from simple advice through to pharmacological replacement and behavioural therapy. None have been shown to be consistently more effective than the other but one may succeed where another has failed. The most successful method seems to be abrupt cessation but this has a high relapse rate. Gradual withdraw may reduce tobacco consumption by is generally ineffective at bringing about cessation.
A minority of patients may stop with simple advice alone, and this is possibly more effective at the time of presentation with a respiratory symptom. Advice should include helpful strategies in stopping such as stopping anybody else smoking within the house, avoiding situations where they automatically smoke, avoiding stress and getting rid of the paraphernalia of smoking (i.e. lighters and ash trays). If simple advice fails then the patient should be encouraged to try again and analyse the stage at which they failed. If they achieved cessation by could not maintain it, then nicotine replacement and behavioural intervention either as an individual or as a group may improve success rates.
Nicotine replacement has been shown to be nearly twice as effective as placebo in achieving long term cessation of smoking. Nicotine is the addictive element in cigarette smoke. When inhaled, it is rapidly absorbed into the blood stream and has half life of about 2 hours. Withdrawal from nicotine may cause unpleasant symptoms such as anxiety, irritability, depression, anger, fatigue, sleep disruption and difficulty concentrating. These effects are most likely to occur within the first week of cessation. Nicotine replacement by chewing gum or transdermal patches may reduce withdrawal symptoms in those who are heavily addicted. These patients usually smoke more than 20 per day, require their first cigarette within 30 minutes of waking and find it difficult to refrain from smoking in non-smoking areas. these patients may require replacement therapy for 6-8 weeks following which they may be weaned off.
Behavioural techniques such as hypnosis may be useful as an adjunct to a smoking cessation program. There is little or no evidence to support the use of acupuncture in smoking cessation.
The cause of expiratory airflow limitation in COPD is
narrowing of the small airways caused by chronic inflammation,
hypertrophy of the airway smooth muscle and enlargement of the
bronchial mucus glands.. The bronchoconstriction that results
differs from asthma in that it is mainly located in the small
airways rather than medium sized ones, it is not due to increased
bronchial wall smooth muscle activity, and it is largely
irreversible, although there may be a degree of reversibility to
bronchodilators in a proportion of patients. The
bronchoconstriction that accompanies inflammation may also
produce a reversible element. This is the rationale behind the
use of bronchodilator agents in the treatment of COPD, and they
are used to maximise airway calibre. However, not all patients
will show a measurable spirometric response to bronchodilating
agents, but most will report symptomatic and functional benefit
despite the lack of objective evidence of improvement.
▀2-adrenoceptor agonists are probably the commonest
prescribed medication in respiratory practice. They are used in
the treatment of asthma and the reversible element of airways
obstruction commonly found in patients with COPD. Although there
are several different types of ▀2-agonist, most are
pharmacologically similar and all are used in a similar fashion.
The two commonest ▀2-agonists prescribed in the UK are
salbutamol (Ventolin«, Ventodisk«) and terbutaline sulphate
(Bricanyl«). Other less common drugs include fenoterol
hydrobromide (Berotec«), rimiterol hydrobromide (Pulmadil«),
pirbuterol (Exirel«), reproterol hydrochloride (Bronchodil«)
and tulobuterol hydrochloride (Brelomax«).
▀2-adrenoreceptor agonists have effects on smooth and skeletal
muscle, which include bronchodilatation, relaxation of the uterus
and tremor. All ▀2-agonists are commonly administered by
inhalation of the drug as an aerosol (either from a metered dose
inhaler (MDI) or a nebuliser) or as powder delivery systems such
as the Turbuhaler from Astra and Diskhaler, Rotacaps and
Accuhaler from Allen & Hanburys.. Salbutamol and terbutaline
are also available as oral slow release tablets, syrups and
intravenous preparations. It was previously thought that only
about 10 per cent of the inhaled aerosol dose actually entered
the lungs, the remainder being swallowed. However, recent studies
using directly labelled drugs suggest a much higher percentage
(up to 20%) can reach the peripheries, and the percentage
distribution can be improved by use of a large volume spacer
device (Volumatic« or Nebuhaler«), which also greatly reduces
the swallowed dose. The maximal therapeutic effect is seen within
15 minutes of inhalation which suggests a local action within the
lungs as the peak plasma concentration of the drug occurs after
about 3 hours after inhalation. In severe acute exacerbations,
▀2 agonists can be given in very high doses using a nebuliser,
and this can also be used by those patients requiring large doses
to obtain symptomatic relief. There is no advantage is giving
▀2-agonists by the intravenous route during acute exacerbations
Recent studies in asthma suggest that better control of symptoms
may be obtained by "as required" rather than regular
dosing, and should be used prophylactically before exertion or
any activity that will precipitate symptoms. There is no evidence
that regular use alters the progress of COPD, but there is some
evidence that regular prolonged use may lessen the acute
bronchodilator effect. Oral salbutamol is now only available as
Volmax«, a specially designed capsule, which osmotically
controls release of the drug by holding it within a core
surrounded by a semi-permeable membrane. Oral preparations of
▀2-agonists are useful in patients unable to use the inhaled
forms of therapy, although with the introduction of spacer
devices and easy to use powder delivery systems, this is
uncommon. Long acting inhaled ▀2-agonists such as salmeterol and
formoterol may be useful when there are troublesome nocturnal or
early morning symptoms. However, the long term effects of these
two agents in patients with COPD has yet to be determined.
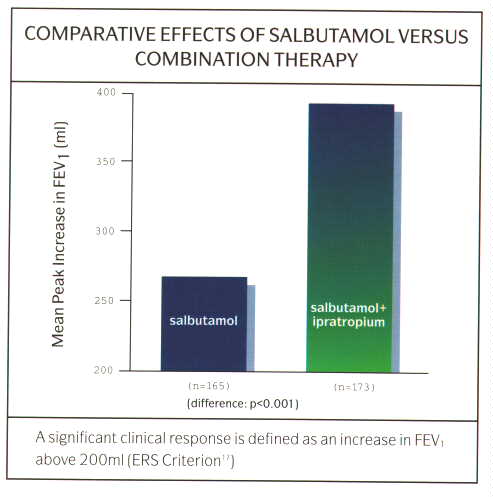
The anticholinergic agents (Ipratropium bromide, Atrovent«, and Oxitropium bromide, Oxivent«) produce bronchodilatation by a different mechanism to the ▀2-agonists. Ipratropium is a non-selective competitive muscarinic acetylcholine receptor antagonist. When given intravenously, is most potent at inhibition of bronchial receptors less so of salivary receptors, and minimal effects on cardiac and urinary bladder receptors. When given by the inhaled route, even in high dosage, the systemic effects are negligible. Its effects are dose related but is a less potent bronchodilator than the ▀2-agonists in asthma but may be equipotent or better in patients with COAD and in the elderly. In patients with asthma and COPD, the combination of ipratropium and a ▀2-agonist may be synergistic (fig 10). The onset of action of ipratropium when given by the inhaled route is slower than that of the ▀2-agonists, being in region of 30-60 minutes but its effects last longer, up to 4 hours. Ideally, a demonstrable improvement in an index of lung function following inhalation of ipratropium should be sought before commencing a patient on treatment with the drug, although in practise, this is rarely done. Side effects are rare as there is little systemic absorption. Some patients may report a dry mouth due to effects on the salivary glands. Drying of bronchial secretions with difficulty with expectoration, glaucoma and acute urinary retention are theoretically possible, however, systemic effects are rarely seen even with high dose nebulised therapy.

Fig. 10: Graph of comparative effects of salbutamol vs combination therapy
Ipratropium can be used the management of patients with COPD as a first line drug or in those whose symptoms are poorly controlled on regular ▀2-agonists. The symptoms in patients with COPD are more persistent than in patients with asthma and their exercise tolerance is limited by their symptoms. Therefore, if the patient needs treatment everyday, then it makes sense to to give the treatment on a regular basis rather than as required as in asthma. There are benefits from combining the two agents in a single inhaler (Combivent MA«, or metered aerosol), as patients will gain the near immediate relief from the salbutamol, then the synergistic activity of the two combined and finally the more prolonged duration of action of the ipratropium (fig 11). All this in the convenience of a single inhaler rather than 2 inhalers. The use of nebulised ipratropium (250-500 Ág 4-6 hourly) in combination with a nebulised ▀2-agonist is common in patients with severe COAD using home nebuliser therapy, and again there are benefits from using a combination of the two agents. This combination is now available as a single unit dose vial (Combivent UDV«), and this has the advantage of providing the same dose of ▀2-agonist and ipratropium in a smaller volume (fig 12). Thus this will reduce nebulisation times by up to half and be more convenient for the patient.
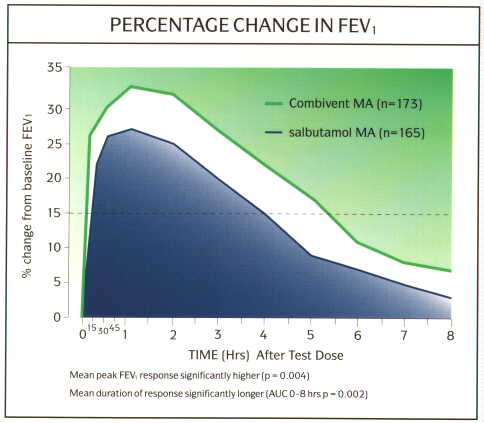
Fig. 11: Graph efficacy and duration of action of Combivent MA vs salbutamol alone
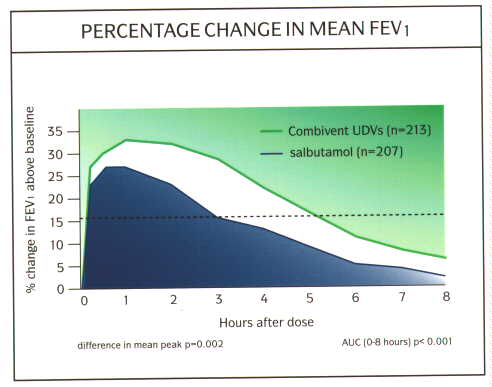
Fig. 12: Graph of efficacy and duration of action of Combivent UDV vs salbutamol
Recently, it has been discovered that the lung muscarinic receptor subtypes (M1, M2 and M3) have different properties, and has promoted the search of selective antagonists to the different types of receptor. The M1 and M3 receptors are responsible for cholinergic induced bronchoconstriction. However, the M2 receptor, which is pre-synaptic, plays a role in a negative feedback loop that actually inhibits further cholinergic activity. Thus, the majority of the bronchodilator effects of anticholinergics are mediated by antagonism of the M1 and M3 receptors, but antagonism of the M2 receptor will not be beneficial. Ipratropium is a non-selective anticholinergic and it blocks all 3 muscarinic receptors. A new anticholinergic agent (Tiotropium bromide, Ba 679 BR), is currently being developed which selectively blocks M1 and M3 receptors and is very long acting. Tiotropium has been found to show improved antimuscarinic activity over ipratropium in animal studies. In patients with COPD, tiotropium has been shown to produce a dose-dependent and prolonged bronchodilator effect that lasts for over 24 hours, and thus may be suitable for once daily dosing. Tiotropium also produces prolonged bronchodilation in asthma. Tiotropium is not currently available commercially , but we await further studies and its arrival with interest
Theophylline has bronchodilator properties and is used in the treatment of asthma and COAD. Despite theophylline being widely available in a large number of proprietary preparations, little is known about its mode of action. Theophyllines are very popular in the USA and on the continent, but some respiratory physicians in the UK still have reservations about its use, mainly because of the high incidence of side effects, particularly at the upper limit of its therapeutic range. However, it still retains an important role in the treatment of acute severe asthma and severe COPD.
Theophylline works as a bronchodilator by the relaxation of bronchial smooth muscle. Several mechanisms have been proposed which include the inhibition of phosphodiesterase to increase intracellular cAMP levels. However, the concentrations of theophylline required to produce measurable increases in cAMP are far outside the levels at which there is a clinical effect. Recently, theophylline has also been shown to have some anti-inflammatory activity, inhibiting the activity of CD4 lymphocytes in vitro and mediator release from mast cells, and can inhibit bronchoconstriction produced by exercise and challenge testing. Theophylline has also been shown to increase the force of contraction of the diaphragm in patients with COAD although this mechanism of action, and any clinical value of this function is still disputed. Theophylline produces bronchodilatation in a concentration dependent manner and continuous therapy can reduce the symptoms of chronic asthma. However, theophylline also reduces dyspnoea in patients with COAD without alteration of their lung function which could be due to a central, cardiovascular of diaphragmatic effect.
Theophylline is well absorbed from the gastrointestinal tract with up to 90-100 per cent bioavailability. Peak levels are achieved within 1-2 hours following ingestion, but this is slowed by the presence of food. Because of the relatively short plasma half-life of theophylline, there are many sustained release preparations available commercially. These all vary as to their bioavailability and the time to peak plasma concentrations. Therefore, once stabilised on one sustained release preparation, patients should not be changed to another without monitoring of plasma levels. Theophylline is mainly metabolised in the liver by demethylation or oxidation using the cytochrome P450 system. Therefore caution needs to be exercised when using other drugs that are also metabolised by the cytochrome system and dosage adjustments need to be made in conjunction with the measurement of plasma levels. Special care should be taken with certain antibiotics as patients with acute infective exacerbations of their airways obstruction may be prescribed them without consideration of the effects on theophylline metabolism. These include the macrolide (e.g. erythromycin) and quinolone (e.g. ciprofloxacin) families of antibiotics which both reduce theophylline clearance to varying degrees. Other drugs that reduce theophylline clearance include cimetidine, allopurinol and propanolol (although this would be a rather unusual therapeutic combination). The rate of metabolism of theophylline is increased substantially in cigarette smokers (the half life can be halved), although may not be significant in those who smoke less than 10/day. Smoking marijuana has a similar effect. Hepatic dysfunction, heart failure and cor pulmonale all reduce the elimination of theophylline.
Theophyllines can is used both in the prophylaxis of chronic asthma and COAD. Sustained release preparations (e.g. Theo-dur«, Phyllocontin«, Uniphyllin«) are preferred because they produce smoother plasma levels throughout a 24 hour period and have better patient compliance. Although a rough guide to total daily dosage is 10-15mg/kg in adults (higher in children) in 2 divided doses for sustained release preparations, the inter-individual variation in metabolism of theophylline and the effect of smoking, drugs and other factors, can make the initial estimate of dosage requirements in an individual patient a hit and miss affair. In acute severe asthma or severe exacerbations of COPD, intravenous theophylline (in the form of aminophylline) is used only when patients fail to respond to the initial treatment of repeated high doses of nebulised 2-agonists and ipratropium bromide with intravenous corticosteroids. Aminophylline should be given initially as a loading dose of 5mg/kg (in patients not already on oral theophylline) as an intravenous infusion over 15-30 minutes, followed by a continuous maintenance infusion. In patients that are taking oral theophylline, the use of intravenous theophylline can cause problems as the plasma level will not be known. The measurement of plasma theophylline levels is seldom available as an emergency, and the patient may be uncertain as to when or if they took their last dose (the compliance with oral theophylline can be very poor because of the high incidence of side effects) or may have taken more than the prescribed dose because of their deteriorating control. Therefore, the administration of a loading dose in this situation may be dangerous as toxic levels may be achieved. The calculation of the maintenance infusion dosage needs to take into account the age of the patient, their smoking history, any concurrent disease and medication. As a rough guide, adult non-smokers should be given 0.4-0.5mg/kg/hour, adult smokers 0.6-0.7mg/kg/hour, and patients with liver dysfunction, heart failure or cor pulmonale 0.2-0.3mg/kg/hour.
One of the factors that limits the usefulness of theophylline is the high incidence of side effects within the therapeutic range and the narrow therapeutic index. As plasma levels exceed 15mg/l (normal therapeutic range 10-20mg/l), the frequency of side effects increases, the most common being a sinus tachycardia, nausea, tremor and indigestion. Indigestion is probably due theophylline increasing gastric secretion and relaxing the gastro-oesophageal sphincter causing gastro-oesophageal reflux. Deaths associated with theophylline toxicity have been reported. These may be due to cardiac toxicity leading to life threatening dysrrhythmias, or associated with neurotoxicity. The mortality from theophylline related seizures approaches 30 per cent. There is no specific treatment, but general measures such as gastric lavage and oral activated charcoal may help reduce plasma levels.
Inhaled corticosteroids are now standard therapy in the management of asthma but their role in the managment of COPD is still not fully established. The use of oral systemic corticosteroids in testing for reversibility of patients with newly diagnosed COPD is well established. An accepted regimen is 30-40mg of prednisolone per day for 10-14 days, with spirometry measured before and directly after the end of the course. Most physicians in the UK would use a short course of oral corticosteroids (again 30-40mg for 7-14 days, with or without weaning) in the management of an acute exacerbation of COPD, although this issue is still debated. Whether or not the corticosteroids should be continued in an inhaled form in those patients who show reversibility is still uncertain. Currently, this is should be decided on an individual patient basis, with regular monitoring of the FEV1. If the maximum FEV1 that was achieved following the course of steroids cannot be maintained with just regular bronchodilator therapy, then inhaled steroids should be tried, and if that is not successful, then maintenance on an oral dose of corticosteroids may be necessary.
There is no evidence that patients who are treated with regular bronchodilators and maintain their usual FEV1 need the protective anti-inflammatory effect of inhaled corticosteroids that asthmatics need. There are studies underway to examine whether inhaled steroids may reduce the rate of decline of FEV1 in patients with COPD, but until these studies are finished, the regular use of inhaled corticosteroids in all patients with COPD cannot be recommended.
The two inhaled drugs commonly available are beclomethasone dipropionate (Becotide«, Becloforte«) and budesonide (Pulmicort«). Both beclomethasone dipropionate (BDP) and budesonide (BUD) are both topically active glucocorticoids that have both anti-inflammatory and immunosuppressive activity. Both drugs can be inhaled either as an aerosol from a metered dose inhaler or nebuliser, or as a powder. Although both drugs rely on their topical activity, there may be significant systemic absorption from the lungs.
The side effects of the two drugs can be divided into those caused by local deposition in the oropharynx, and those caused by systemic absorption. Patients may complain of a sore or dry throat, and occasionally, oropharyngeal candidiasis may occur. This can be prevented to some extent by gargling and rinsing of the mouth after inhaling the drug and the use of a large volume spacer device (Volumatic«, Nebuhaler«) to reduce oropharyngeal deposition. Systemic side effects are seen in high dosage. Other systemic effects of inhaled steroids that have reported include increased easy bruising and dermal thinning and possibly osteoporosis and cataract formation. It should be noted that systemic effects may be common with BDP rather than BUD because of the faster systemic clearance of BUD with the formation of inactive metabolites.