FORMULATION AND EVALUATION OF BUCCAL FILM CARVEDILOL
R.Venkatalakshmi*1, Yajaman Sudhakar2, M.Mohan Varma1,
C. Madhuchudana Chetty3, C.Sasikala1
Shri Vishnu College of Pharmacy, Bhimavaram,West Godavari Dt, Andhrapradesh, India*1.
Govt. Polytechnic for Women, Kadapa, Andhrapradesh, India2Annamacharya College of Pharmacy, Rajampet, Andhrapradesh, India3.
SUMMARY:
Buccal delivery is considered to be an important alternative to the per oral route for the systemic administration of drugs. The aim of the study was to develop and evaluate mucoadhesive buccal films of Carvedilol for systemic delivery. This paper explains the fabrication of new bilayered films consisting of a drug containing mucoadhesive layer and a drug free backing layer. The films were fabricated by a casting technique with different polymer combinations and were evaluated for in vitro release, thickness, microenvironment pH, drug content uniformity, ex vivo residence time, uniformity of weight, moisture absorption studies. The physicochemical interactions between Carvedilol and polymers were investigated by FTIR Spectroscopy. The present buccal formulation can be an ideal system to improve the bioavailability of the drug by avoiding hepatic first-pass metabolism.
Key words: Oral buccal mucosal delivery, Carvedilol, bilayered buccal films, in vitro release.
INTRODUCTION:
Carvedilol is a β-adrenergic blocking agent with α1-blocking activity, indicated in the treatment of mild to moderate congestive heart failure (CHF) and is used for treating high blood pressure. When combined with other treatments for heart disease among patients with recent heart attacks, Carvedilol can reduce the risk of a second heart attack by 40% and increase survival among patients with congestive heart failure. For high blood pressure and congestive heart failure, the dose may range from 6.25mg and 3.25mg twice daily to a maximum of 25mg and 80 mg twice daily1. It is rapidly and extensively absorbed, but bioavailability is low due to a significant degree of first-pass metabolism. Following oral administration, the apparent mean terminal elimination half-life of Carvedilol generally ranges from 7 to 10 hours. From both, physicochemical (low molecular weight 406.5 g/mol, low dose 6.25 mg) and pharmacokinetic (absolute bioavailability about 25-35 % ) views, Carvedilol is considered to be suitable for buccal delivery. The log PC (partition coefficient) value for Carvedilol is about 3.967. It indicates that Carvedilol has sufficient lipophilicity to pass through the buccal membrane, so that the bioavailability of Carvedilol is increased and extended.
The buccal mucosa has been investigated for local drug therapy and the systemic delivery of therapeutic peptides and other drugs that are subjected to first-pass metabolism or are unstable within the rest of the gastrointestinal tract. The mucosa of the oral cavity presents a formidable barrier to drug penetration, and one method of optimizing drug delivery is by the use of adhesive dosage forms. The buccal route has the advantage of allowing excellent accessibility, reasonable patient acceptance and compliance, avoids first-pass metabolism and involves a relatively robust mucosa. The buccal mucosa offers several advantages for controlled drug delivery for extended periods of time. The mucosa is well supplied with both vascular and lymphatic drainage, the first-pass metabolism in the liver and pre-systemic elimination in the gastrointestinal tract are avoided [1-5].
MATERIALS AND METHODS:
Carvedilol and HPMC (15 Cps) were received as gift samples from Dr. Reddys Labs., Hyderabad and Shasun Pharmaceuticals, Pondicherry., India. All other chemicals were of analytical grade.
CALIBRATION CURVE:
Carvedilol is dissolved in 50ml of methanol and 50 ml of phosphate buffer pH 6.8 was obtained (Stock I). From that 10 ml was taken and made up to 100 ml with phosphate buffer pH 6.8 (Stock II). From the above solution 1,2,3,4,5,6 ml was taken and made up to 10 ml with phosphate buffer pH 6.8 in the concentration range of 10-60 μg/ml. Absorbance was measured at λmax 241 nm with a UV-VIS spectrophotometer (Lab India). The graph is shown in Fig1.
COMPATIBILITY STUDIES (IR SPECTRAL ANALYSIS):
The compatibility studies were carried out at room temperature by Fourier Transform infra red spectroscopy to determine the interaction of Carvedilol with Polymers (HPMC 15Cps, SCMC and Ethyl Cellulose). IR spectra of drug, polymers and combination of drug and polymers are shown in fig-2 to 8.
PREPARATION OF BILAYERED FABRICATED BUCCAL FILM:
SECONDARY LAYER (BACKING LAYER).
For preparing a formulation, a glass petri plate of 7.5 cm diameter was used as a casting surface. Initially, backing membrane of ethyl cellulose was fabricated by slowly pouring a solution containing 750 mg of ethyl cellulose and 4 drops Propylene glycol in 13 ml Dichloromethane and Methanol (1:1) to the glass petri plate. Mercury was used as a substrate and air dried for 9 hrs.
PRIMARY LAYER (MUCOADHESIVE LAYER CONTAINING DRUG).
Initially, HPMC E15 polymer 750mg was dissolved in 10 ml of distilled water under constant stirring till clear solution was obtained. Then to this solution, 4 drops of propylene glycol was added and kept for 4 hrs for swelling. To this solution Carvedilol 87.890 mg required for 14 films (2cm diameter of 1 film=6.25mg) was dissolved in 3 ml of DMSO and was added by stirring. The resultant solution was then poured on the preformed backing layer of ethyl cellulose secondary layer) and allowed to dry undisturbed for 18 h at 50°C in the oven. The dried bilayered patch was cut into discs of 2 cm diameter. Similarly formulations 2 to 6 were prepared by using different concentrations of HPMC 15Cps, SCMC. The compositions of films are reported in table no 1[6-11].
DRUG CONTENT UNIFORMITY:
Uniformity of drug content was determined according to the following procedure. Three randomly selected films of each batch were weighed accurately and dissolved in 50 ml of methanol and stirred continuously for 1 h on a magnetic stirrer. The volume was made up to 100 ml with phosphate buffer (pH 6.8) and then 0.1 ml was transferred to 10ml volumetric flask and the volume was adjusted with phosphate buffer (pH 6.8). The absorbances were measured on a UV/Vis spectrophotometer at 241 nm (Lab India). Concentrations of Carvedilol were calculated from a standard calibration curve of Carvedilol and the results are reported in table no 2.
.
UNIFORMITY OF WEIGHT OF THE FILM:
Individual films were directly weighed on the electricall balance and the average weight was calculated and the results are reported in table no 2.
MICROENVIRONMENT pH:
The microenvironment pH of the prepared buccal bioadhesive Carvedilol films was determined to evaluate the possible irritation effects on the mucosa. The films were left to swell in 1 ml of distilled water (pH 6.8) in small beakers, and the pH was measured after 1 h by placing the electrode in contact with the microenvironment of the swollen films allowing it to equilibrate for 1 minute. The average pH of five determinations are reported in table no 2.
THICKNESS TESTING:
The thickness of ten randomly selected films from every batch were determined using a standard screw gauge and the results are reported in table no 2.
IN VITRO DRUG RELEASE STUDIES:
The drug release from buccal film was studied using the USP 28, type II dissolution test apparatus. The phosphate buffer (PH 6.8), 500 ml of was used as the dissolution medium, at 37.0 ± 0.5ºC, and a rotation speed of 50 rpm was used. Ethyl cellulose layer of the buccal patch was attached to the paddle with water proof adhesive tape. Samples (5 ml) were withdrawn at 0.5, 1, 1.5,2,3,4,5 hr intervals and replaced with fresh medium. The samples were filtered through 0.45-μm Whatman filter paper and analyzed. The drug concentrations were determined spectrophotometrically at the wavelength of 241 nm. The experiments were performed in triplicate, and average values are reported in table 3 and Fig 9 and 10.
MOISTURE ABSORPTION STUDIES:
Moisture absorption studies were performed in accordance with a procedure reported earlier [5-9]. Briefly, 5% w/v agar in distilled water, which in hot condition was transferred to Petri plates and allowed to solidify. Then 6 films from each formulation were weighed and placed over the surface of the agar and left for 3 hr at 37ºC in incubator and the hydrated film was weighed again. The percentage of moisture absorbed was calculated using the formula: [12-13]
% Moisture absorbed
= [(Final weight - Initial weight)/Initial weight] × 100
The results are reported in table no 2.
EX VIVO RESIDENCE TIME:
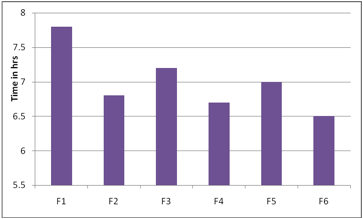
The ex vivo mucoadhesion time was studied (n = 3) after application of patches on freshly cut porcine buccal mucosa. The fresh porcine buccal mucosa was fixed in the inner side of a beaker, about 2.5 cm from the bottom, with water proof adhesive tape. Mucoadhesive layer containing drug of each patch was wetted with 1 drop of phosphate buffer (pH 6.8) and pasted to the porcine buccal mucosa by applying a light force with a fingertip for 30 seconds[12,15]. The beaker was filled with 200 ml of phosphate buffer (pH 6.8) and was kept at 37ºC ± 1ºC. After 2 minutes, a 50-rpm stirring rate was applied to simulate the buccal cavity environment, and the film adhesion was monitored for 8 hours. The time required for the patch to detach from the porcine buccal mucosa was recorded as the mucoadhesion time and the results were reported in table no 2 and fig 11.
RESULT AND DISCUSSION:
The results revealed that the release of drug depends on the polymer type as well as its concentration. Film containing HPMC E15 alone (F1 & F3) released the maximum drug. The plots of log cumulative percent drug retained against time (Fig. II) were found to be linear. This indicated that drug diffusion from these buccal films followed a first order kinetics. The rank order of the drug release from different formulations is F1>F3>F2>F6>F4>F5. Formulation F1 showed 89.51% drug release at the end of 2 hr followed by F2 (89.46%), F3 (80.41%) and F4 (65.23%). The formulation F5 showed 59.09%, F6 (72.09%).
The bioadhesive strength of the formulation was found to be dependent on the type of polymers used. Results obtained showed that the formulation F2 exhibited maximum bioadhesion strength. The order of bioadhesion strength for all the formulations F2>F1>F4>F3>F5>F6>. The content uniformity of all the films revealed the drug was uniformly distributed throughout the films.
The surface pH was determined in order to investigate the possibility of any side effects, in the oral cavity. Acidic or alkaline pH is bound to cause irritation to the buccal mucosa. Attempt was made to keep the surface pH close to the neutral pH. The surface pH of all the formulations was found to be within 1.5 units of neutral pH. Hence it is assumed that these formulations cause no irritation in the oral cavity.
Thickness of films was found to be in the range of 0.73mm to 1.56 mm for all the films. The rank order was F5>F6>F3>F1>F6>F2.The results are shown in Table No.2.
The results obtained in the present investigation indicate that the films exhibited satisfactory physical and mechanical properties. F1, F2 and F3 showed good acceptability. Formulation F4, F5, F6 delivered 78.64,75.27, 86.06% of drug release at the end of the 4 hours. To improve the release of drug from these films, necessary alteration in composition could be done and can be used as a good device for buccal delivery of Carvedilol.
The present study was a satisfactory attempt to develop erodible buccoadhesive films, which will overcome the inherent drawbacks associated with conventional drug delivery of Carvedilol and will have an improved bioavailability, fast therapeutic action and patient compliance. Further, in vivo release studies need to be carried out on suitable animal models in order to establish the in vitro – in vivo correlation. Also, there is challenge for manufacturer to device suitable manufacturing process to enable large-scale production and willingness of the pharmaceutical industry to take up potential candidates so as to offer an alternative to conventional drug therapy.
CONCLUSION:
The present study indicates enormous potential of erodible mucoadhesive buccal films containing carvedilol for systemic delivery with an added advantage of circumventing the hepatic first pass metabolism. The results of the study show that therapeutic levels of Carvedilol can be delivered buccally. It may be concluded that the films containing 6.25 mg Carvedilol in /v HPMC 15cps in different concentrations show good swelling, a convenient residence time and promising controlled drug release, thus seems to be a potential candidate for the development of buccal film for effective therapeutic use. The further studies are required to enhance the residence time of the buccal films in the oral cavity, so that the potential therapeutic benefit can be received. In vivo studies need to be designed and executed to substantiate further in- vitro in-vivo correlation.
REFERENCE:
1.Okamoto H, Taguchi H, Iido K, Danjo K. Development of polymer film dosage forms of lidocaine for buccal administration, I: penetration rate and release rate. J Control Release. 2001;77:253Y260.
2.Vishnu M. Patel, Bhupendra G. Prajapati and Madhabhai M. Patel. Effect of Hydrophilic Polymers on Buccoadhesive Eudragit Patches of Propranolol Hydrochloride Using Factorial Design. AAPS PharmSciTech 2007; 8 (2) Article 45.
3. Chandra Sekhar K, Naidu K. V. S, Vamshi Vishnu Y, Ramesh Gannu, Kishan V and Madhusudan Rao Y. Transbuccal Delivery of Chlorpheniramine Maleate from Mucoadhesive Buccal Patches. Drug Delivery, 2008, 15:185–191.
4. Sanjay Singh, Rajeev Soni, Manoj Kumar Rawat, Achint Jain, Shripad Bheemrao Deshpande, Sanjeev Kumar Singh, and Madaswamy Sona Muthu C. In Vitro and in Vivo Evaluation of Buccal Bioadhesive Films Containing Salbutamol Sulphate. Chem. Pharm. Bull. 2010, 58(3) 307—311.
5. Hoogstraate A. J and Wertz P. W. Drug delivery via the buccal mucosa. PSTT. 1998, 17:309–316.
6. Vishnu M. Patel, Bhupendra G. Prajapati and Madhabhai M. Patel Effect of Hydrophilic Polymers on Buccoadhesive Eudragit Patches of Propranolol Hydrochloride Using Factorial Design. AAPS PharmSciTech 2007; 8 (2) Article 45.
7. Shojaei, A.H. Buccal Mucosa as A Route for Systemic Drug Delivery. J. Pharm. Pharmaceut. Sci., 1998, 1, 15-30.
8. Giunchedi, P, Juliano, C, Gavini, E, Cossu M, Sorrenti M. Formulation and in vivo evaluation of chlorhexidine buccal tablets prepared using drug loaded chitosan microsphears. Eur. J. Pharm. Biopharm., 2002, 53, 233-9.
9. Khanna R, Agarwal S P, Ahuja A. Preparation and evaluation of muco adhesive buccal films of clotrimazole for oral candida infections. Int. J. Pharm., 1996, 138, 67-73.
10. Nagai, T, Konishi R. Buccal/gingival drug delivery systems. J.Control. Release, 1987, 6, 353-60.
11. Burgalassi S, Panichi L, Saettone M.F Jacobsen J Rassing M.R. Development and in-vitro/in vivo testing of mucoadhesive buccal patch releasing benzydamine and lidocaine. Int. J. Pharm.,1996, 133, 1-7.
12. Reinhold A, Hans P.M. Evaluation of laminated mucoadhesive patches for buccal drug delivery. Int. J. Pharm., 1989, 49, 231-40.
13.Wong C.F, Yuen, K.H, Peh K.K. Formulation and evaluation of controlled release eudragit buccal patches. Int. J. Pharm., 1999,178, 11-22.
Table1. Composition of bilayered buccal films of Carvedilol
Component |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
Primary layer (Mucoadhesive layer) |
||||||
Carvedilol (mg) |
87.890 |
87.890 |
87.890 |
87.890 |
87.890 |
87.890 |
HPMC E15 (mg) |
750 |
500 |
1000 |
- |
- |
- |
Sodium Carboxy Methyl Cellulose |
- |
- |
- |
750 |
500 |
1000 |
Propylene glycol |
4 drops |
4 drops |
4 drops |
4 drops |
4 drops |
4 drops |
Distilled water(ml) |
15 |
15 |
25 |
25 |
15 |
15 |
DMSO(ml) |
3 |
3 |
3 |
3 |
3 |
3 |
Secondary layer (Backing layer) |
||||||
Ethyl cellulose(mg) |
750 |
1000 |
1000 |
750 |
1000 |
1000 |
Propylene glycol |
4 drops |
4 drops |
4 drops |
4 drops |
4 drops |
4 drops |
Dichloromethane: Methanol(ml) 1:1 |
13 |
13 |
13 |
13 |
13 |
13 |
Fig 1. Calibration curve of Carvedilol.
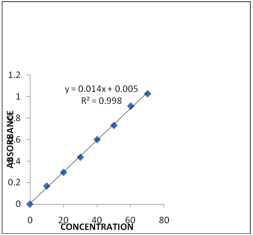
Table 2: Physicochemical Characteristics of the Prepared Buccal Bioadhesive Films of Carvedilol:
Formulation code |
Film weight mg. |
Surface pH |
(Assay)% Drug Content w/w ±(SD) |
Film thickness mm ±(SD) |
Swelling index (3h) |
Ex vivo residence time (h) |
F1 |
366 ± 4.26
|
6.13±2.11 |
102.02±2.26 |
1.13±0.08 |
40.57±2.33 |
7.8±1.20 |
F2 |
233±3.32 |
6.05±1.22 |
108.08±1.01 |
0.73±1.11 |
34.28±1.22 |
6.8±2.12 |
F3 |
514±3.87 |
7.16±3.22 |
104.04±1.22 |
1.13±1.09 |
44.97±3.33 |
7.2±1.11 |
F4 |
367±3.12 |
6.84±4.22 |
98.98±2.31 |
0.99±1.12 |
55.89±1.11 |
6.7±4.33 |
F5 |
449±2.88 |
6.84±1.28 |
103.03±3.22 |
1.56±1.22 |
50.00±4.22 |
7.0±2.11 |
F6 |
565±1.22 |
6.83±2.44 |
109.09±4.33 |
1.46±0.22 |
38.96±3.22 |
6.5±2.22 |
Formulation Code |
|
|
Percentage Drug release* ±SD |
|||
30 minutes |
60 minutes |
90 minutes |
120 minutes |
180 minutes |
240 minutes |
|
F1 |
44.412±3.03 |
67.98±12.96 |
72.81±11.17 |
89.51±14.02
|
92.31±14.49 |
102.57±4.63 |
F2 |
34.064±2.89 |
65.99±7.2 |
77.93±0.95 |
89.465 ±5.09
|
94.185±5.15 |
98.83±6.96 |
F3 |
38.424±4.05
|
66.86±7.96 |
71.11±6.6 |
80.414±8.71 |
91.825±3.02 |
101.18±2.42 |
F4 |
33.09±1.212 |
50.65±0.98 |
58.42±0.60 |
65.23±5.65 |
71.89±4.09 |
78.64±1.20 |
F5 |
31.29±0.90 |
45.27±1.37 |
53.70±1.22 |
59.09±6.99 |
64.78±8.78 |
75.27±3.93 |
F6 |
35.08±5.26 |
43.73±03.12 |
65.56±5.75 |
72.09±5.13 |
78.07±0.89 |
86.06±5.31 |
Table-3: In vitro dissolution profile data for Carvedilol buccal patches for all the formulations:
Fig 2: IR Spectra of Carvedilol
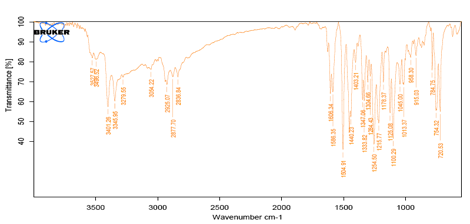
Fig 3: IR Spectra of HPMC 15CPS
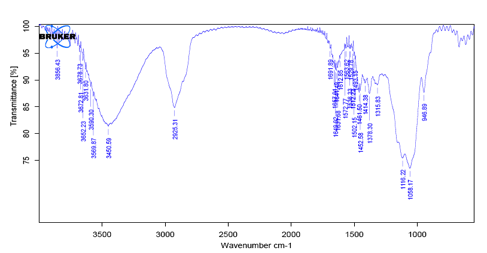
Fig 4: IR Spectrum of SCMC.
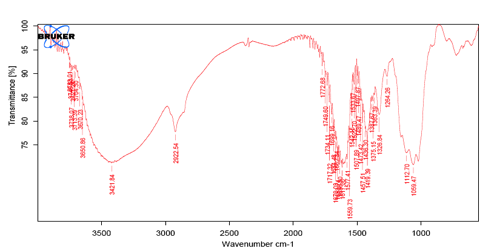
Fig 5: IR Spectrum of Ethyl cellulose
Fig 6: IR Spectrum of Carvedilol and HPMC 15CPS
Fig 7: IR Spectrum of Carvedilol and SCMC 
Fig 8: IR Spectrum of Carvedilol and Ethyl cellulose
Fig 9: In vitro cumulative % drug release profiles from different bucoadhesive films of Carvidilol
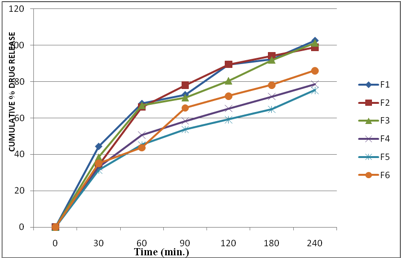
Fig10. Comparison of maximum % drug released in in vitro dissolution studies of Carvedilol Buccal Films (6.25mg/patch) at 240 minutes: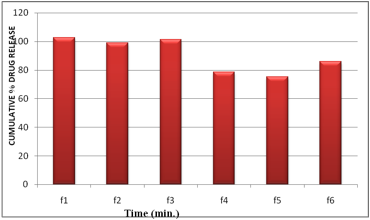
Fig 11: Exvivo Residence Time of Carvedilol Buccal Films:
Received March 2011. Published March 2011.
Click
on these links to visit our Journals:
Priory Bookshop | Search for papers and articles
| Psychiatry
On-Line
Dentistry
On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages copyright ©Priory Lodge Education Ltd 1994-


