Browse through our Journals...
Inhaler Aerosols - a Comparative Assessment of Quality Control Parameters
Sonali R. Naikwade, Shipla Balakrishnan, Amrita N. Bajaj,
C. U. Shah College of Pharmacy, S.N.D.T. Woman’s University, Juhu-Tara Road,
Santacruz (W), Mumbai-400049, Maharashtra, India.
Summary:
Inhaler aerosols are pressurized systems intended for local action in the respiratory tract. Various types of inhalers such as nebulizers, metered dose inhalers (MDIs) and dry powder inhalers (DPIs) are available. A unique feature of MDIs is propellant whose properties like vapour pressure, viscosity and density affect the performance of inhalation aerosols. Inspite of their intricate design, variations in the amount of dose delivered through these devices have been reported. Hence a comparative study was undertaken to assess the uniformity and accuracy of dosage delivery from metered dose inhalers (MDIs). Marked differences were observed in the spray pattern, content uniformity and respirable fraction of the dose emitted from MDI devices.
Keywords:
Comparative study; metered dose inhalers; performance of aerosols
Introduction
Inhalation aerosols are fine suspensions or dispersions of solid particles in a gas, intended for administration into the respiratory tract. Aerosol route of administration targets drugs directly to lungs and offers advantages of bypassing the first pass elimination, reducing the dosage frequency and minimizing the extent of adverse drug reactions (Ashurst, et al., 2000; Grossman, 1994; Khilnani and Banga, 2004). This in turn, improves the therapeutic effect-toxicity ratio.
Therapeutic performance of aerosols is affected by various factors such as actuator tube design, orifice diameter, concentration of surfactant in the system, vapour pressure of propellants, efficiency of valve crimping and particle size of the plume emerging from the inhaler. Unique feature of this dosage form is presence of propellants, whose properties like flash point, viscosity and density also modify the aerosol performance (Sciarra and Cutie, 1987).
MDIs are pressurized packs and many quality control tests are necessary to ensure their proper performance. Aerosols are evaluated based on series of tests carried out during the formulation development and finished product testing stages (Brambilla, et al., 1999). Standard tests for stability, purity and dose uniformity as performed on conventional dosage forms are also applicable to aerosols however MDIs require special considerations during manufacturing, packing and testing procedures because of the pressurised canisters (Newman and Clarke, 1992; Sciarra and Cutie, 1987). In-process checks regarding the active drug concentration in the product, level of dispersion and extent of aerosolization are also essential. Inspite of their intricate design, variations in the amount of dose delivered through these devices have been reported (Herzka and Pickthall, 1961). In order to restrict and control these variations, MDIs should be subjected to strict quality control testing. Hence a comparative study was undertaken to assess the dose uniformity and accuracy of dosage delivery from several marketed metered dose inhalers (Leon Lachman, 1986).
Standard tests for metered dose inhalers as per I.P. includes content per spray, number of deliveries per container, particle size distribution, pressure test and leak test (Anonymous, 1996). B.P. specifies tests viz. content per spray, number of deliveries per container, particulate matter , water content and deposition of emitted dose (Anonymous, 1993). U.S.P. specifies following tests: content per spray, pressure test and leak test (Anonymous, 2000). Tests like content per spray and particle size distribution which affect aerosol performance are included in the Pharmacopoeia. I.P monograph also specifies standards for propellants, active ingredients, containers, valves and actuators. However an important test like deposition of emitted dose that governs the clinical efficacy of metered dose inhalers is to be included in I.P. and also in U.S.P.
MATERIALS AND METHODS
Materials
Out of the popular brands of metered dose inhaler products available in Indian market, six MDIs, two each of salbutamol, beclomethasone and budesonide were selected and coded as A, B, C, D, E and F.
Methods
Selected aerosol products were subjected to a series of tests to determine dimensions of container, total drug content, number of deliveries per container, leak test and leakage rate as listed above. Physicochemical characteristics of aerosol products were determined based on quality control tests as mentioned in monographs: Spray pattern was determined by impinging the spray from MDI on to a TLC plate containing silica gel- dye mixture from a distance of 3 cm as shown in figure 1.
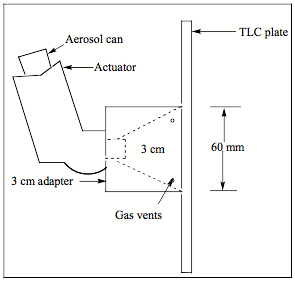
Figure 1: Set up to determine spray pattern using thin layer chromatography
Particle size distribution of aerosol products was determined using optical microscopy. Average weight per metered dose is test similar to weight variation test performed for tablets and capsules. Average weight of 5 deliveries sprayed from each device was estimated. Content per spray was determined by spectrophotometric analysis of the active ingredient and checked for compliance with the labeled claim. In order to perform content uniformity test, ten doses were individually assessed for their active drug content by respective spectrophotometric methods of analysis. To check uniformity of dosage delivery of initial, middle and final doses, every 1st, 25th, 50th, 100th, 150th, 200th and 225th dose sprayed from the device was checked for their drug content as shown in figure 3. Deposition of emitted dose was determined using the Twin Stage Impinger Apparatus B.P. (Copley scientific Limited) (Labiris and Dolovich, 2003; Leach, et al., 2002; Moren, 1978). It comprised two stage reservoirs (figure 2) - stage I, representing the amount of dose deposited in the oropharyngeal region and stage II (also termed as respirable fraction), representing the amount of drug deposited in lungs (Feddah, et al., 2000).
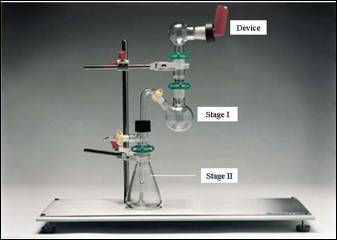
Figure 2: Twin Stage Impinger Apparatus B.P.
Retention on the adapter was estimated by analyzing the adapter and the valve rinsing for drug content. Flame extension was determined by spraying the product on to an open flame from a distance of 18 cm and measuring the flame extension produced with a ruler. Water content was determined using coulometer (Make- Metrohm model 684KF).
RESULTS AND DISCUSSION
Although the inhalation aerosol formulations met with standards mentioned in I.P monograph, when results of quality control parameters were statistically analyzed, high standard deviations were obtained. Spray pattern is an important test for evaluating the actuator and the valve performance. It was indicated by spots formed on the silica gel plate which varied from 8-12 mm in diameter for salbutamol. Particle size of droplet plume emerging from the orifice is an important parameter which influences the deposition of inhalation aerosols in lungs. Efficacy of bronchial delivery is dependent on the effective aerodynamic diameter of particles. The optimum diameter for deposition in lungs has been reported to be between 2-10 µ. Interesting observations were made for steroidal inhalers, where spots ranged from 18-25 mm in diameter. It was observed that 97-98% of particles were ≤ 2 µ and very few of them were in the range > 2-5 µ.
Significant standard deviations were obtained for average weight per metered dose. Product A showed a value of 88.04 ± 0.60, product B, C, D, E showed weight/metered dose as 87.16 ± 1.68, 86.95 ± 0.82, 85.38 ± 0.76, 87.20 ± 1.76, whereas product F showed a least value of 71.38 ± 0.26.
Content per spray determines the amount of active ingredient of a definite number of deliveries sprayed from the canister. High standard deviations were obtained for content per spray, where the drug content varied form 105.9% ± 0.90 for product A to 107.5% ± 3.09 for product E. Content uniformity test determines the amount of active ingredient from an individual dose sprayed from the container. Very high standard deviations ranging from ± 5.8 to ± 10.2 were also observed for content uniformity test. In order to check variabilities in initial and final doses emitted from MDIs, the study was extended to determine the drug content from the 1st, 25th, 50th, 100th, 150th, 200th and 225th doses sprayed from the inhaler device. As shown in figure 3, maximum dosage uniformity was observed from the 50th- 150th dose, whereas the initial and later doses showed the evidence of tapering.
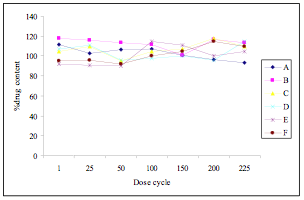
Figure 3: Content uniformity of the 1st, 25th, 50th, 100th, 200th and 225th dose
Deposition of emitted dose is a critical parameter that affects the clinical efficacy of inhalation aerosol (Lipworth and Clark, 1997; Newman, et al., 1981). When a dose is sprayed from the container, some of the larger droplets formed as a result of atomization tend to deposit on the inner surface of the mouthpiece of the adapter. This represents the fraction that is not available for inhalation and should be restricted to the minimum. Products were found to be highly retained on the adapter (6- 13%), thereby minimizing the respirable fraction. Respirable fraction of the emitted dose was found to vary from 34-55% (respirable fraction for product A, B, C, D, E and F were 55.80, 52.29, 46.71, 49.30, 54.86 and 34.06% respectively). Flame extension test indicates the flammability of the propellants used in the formulation. Propellants used in the aerosol formulations were found to be nonflammable as insignificant flame extensions (< 3 cm) were produced by products. Water content is an important test that determines the stability of aerosol formulation. Trace amounts of water can hamper the stability of suspension formulations by causing aggregation of suspended particles leading to sedimentation and Ostwald ripening. Very high standard deviations were also observed for water content where values ranged between ± 1.21 and ± 6.47. This may lead to instability of suspension resulting in non-uniformity of the emitted dose.
CONCLUSION
There is an imminent need to include more stringent tests for evaluation of MDIs like spray pattern, average weight per metered dose, content uniformity, deposition of emitted dose and water content in addition to standard tests mentioned in the Pharmacopoeia. Variations in the aerosol performance may be attributed to the formulation design and packaging system used for MDIs hence great attention is needed in design, development and evaluation of aerosol formulations.
REFERENCES
Anonymous. 1993. British Pharmacopoeia. p 740-742.
Anonymous. 1996. The Pharmacopoeia of India. 3rd ed., New Delhi: Controller of Publications. p 25-27.
Anonymous. 2000. United States Pharmacopoeia. United states pharmacopoeial convention Inc. p 1895-1912.
Ashurst, I, Malton, A, Prime, D, Sumby, B. (2000) Latest advances in the development of dry powder inhalers. Pharmaceutical Science and Technology Today. 3, (7):246-256.
Brambilla, G, Ganderton, D, Garzia, R, Lewis, D, Meakin, B, Ventura, P. (1999) Modulation of aerosol clouds produced by pressurised inhalation aerosols. International Journal of Pharmaceutics. 186:53-61.
Feddah, M R, Brown, K F, Gipps, E M, Davies, N M. (2000) In vitro characterization of metered dose inhaler versus dry powder inhaler glucocorticoid products: influence of inspiratory flow rates. Journal of Pharmacy and Pharmaceutical Sciences. 3, (3):317-324.
Grossman, J. (1994) The evolution of inhaler technology. Journal of Asthma. 31:55-64.
Herzka, A, Pickthall, J. 1961. Pressurised packaging (aerosols). 2nd ed., London: Butterworth. p 167-169.
Khilnani, G C, Banga, A. (2004) Aerosol Therapy. Journal of Indian Academy of Clinical Medicine. 5, (2):114-123.
Labiris, N R, Dolovich, M B. (2003) Pulmonary drug delivery. Part II: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. British Journal of Clinical Pharmacology. 56, (6):600-612.
Leach, C L, Davidson, P J, Hasselquist, B E, Boudreau, R J. (2002) Lung depostion of hydrofluoroalkane-134a beclomethasone is greater than that of chlorofluorocarbon fluticasone and chloroflourocarbon beclomethasone. Chest. 122, (2):510-516.
Leon Lachman, H L. 1986. Theory and Practice of Industrial Pharmacy. 3rd ed. p 586.
Lipworth, B J, Clark, D J. (1997) Lung delivery of salbutamol by dry powder inhaler (Turbuhaler®) and small volume antistatic metal spacer (Airomir® CFC-free MDI plus NebuChamber®). European Respiratory Journal. 10: 1820–1823.
Moren, F. (1978) Drug deposition of pressurised inhalation aerosols II. Influence of vapour pressure and metered volume. International Journal of Pharmaceutics. 1: 213-218.
Newman, S P, Clarke, S W. 1992. Inhalation devices and techniques in asthma. 3rd ed., London: Chapman & Hall. p 469-505.
Newman, S P, Pavia, D, Moren, F, Sheahan, N F, Clarke, S W. (1981) Deposition of pressurized aerosols in the human respiratory tract. Thorax. 36: 52-55.
Sciarra, J, Cutie, J. 1987. Pharmaceutical aerosols. In Leon Lachman, H L, editor Theory and Practice of Industrial Pharmacy, 3rd ed. p 589-618.
Copyright Priory Lodge Education Limited 2008
Firts Published March 2008
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


